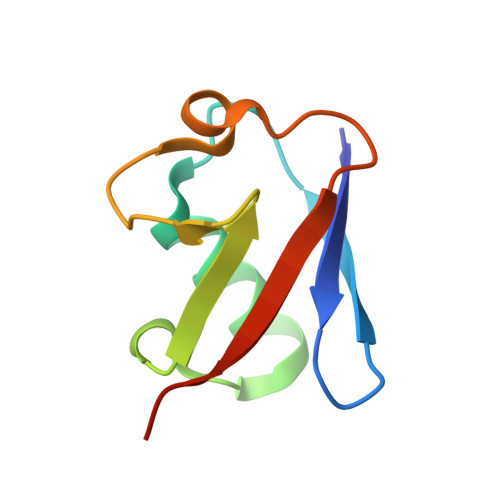
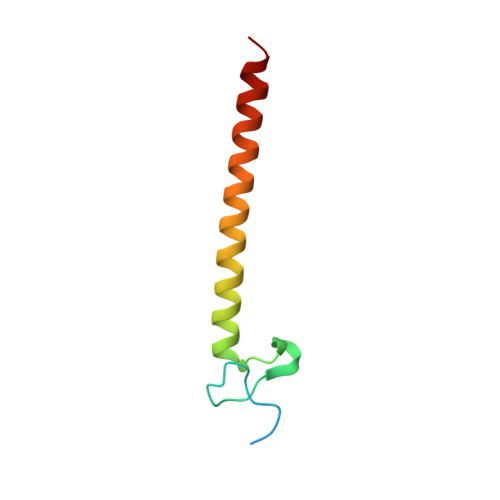
Structural basis for ubiquitin recognition and autoubiquitination by Rabex-5
Lee, S., Tsai, Y.C., Mattera, R., Smith, W.J., Kostelansky, M.S., Weissman, A.M., Bonifacino, J.S., Hurley, J.H.(2006) Nat Struct Mol Biol 13: 264-271
- PubMed: 16462746
- DOI: https://doi.org/10.1038/nsmb1064
- Primary Citation of Related Structures:
2FID, 2FIF - PubMed Abstract:
Rabex-5 is an exchange factor for Rab5, a master regulator of endosomal trafficking. Rabex-5 binds monoubiquitin, undergoes covalent ubiquitination and contains an intrinsic ubiquitin ligase activity, all of which require an N-terminal A20 zinc finger followed immediately by a helix. The structure of the N-terminal portion of Rabex-5 bound to ubiquitin at 2.5-A resolution shows that Rabex-5-ubiquitin interactions occur at two sites. The first site is a new type of ubiquitin-binding domain, an inverted ubiquitin-interacting motif, which binds with approximately 29-microM affinity to the canonical Ile44 hydrophobic patch on ubiquitin. The second is a diaromatic patch on the A20 zinc finger, which binds with approximately 22-microM affinity to a polar region centered on Asp58 of ubiquitin. The A20 zinc-finger diaromatic patch mediates ubiquitin-ligase activity by directly recruiting a ubiquitin-loaded ubiquitin-conjugating enzyme.
- Laboratory of Molecular Biology, National Institute of Diabetes and Digestive and Kidney Diseases, US National Institutes of Health (NIH), Bethesda, Maryland 20892, USA.
Organizational Affiliation: