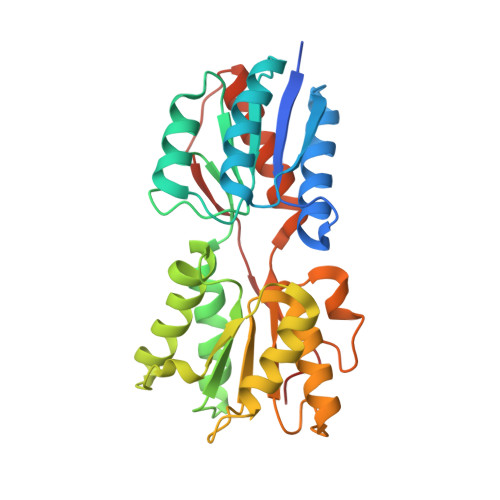
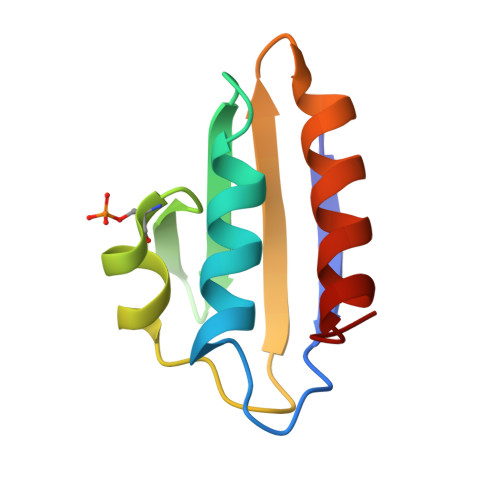
Structural analysis of B. subtilis CcpA effector binding site.
Chaptal, V., Gueguen-Chaignon, V., Poncet, S., Lecampion, C., Meyer, P., Deutscher, J., Galinier, A., Nessler, S., Morera, S.(2006) Proteins 64: 814-816
- PubMed: 16755587
- DOI: https://doi.org/10.1002/prot.21001
- Primary Citation of Related Structures:
2FEP - Laboratoire d'Enzymologie et Biochimie Structurales, CNRS FRE 2930, Gif-sur-Yvette, France.
Organizational Affiliation: