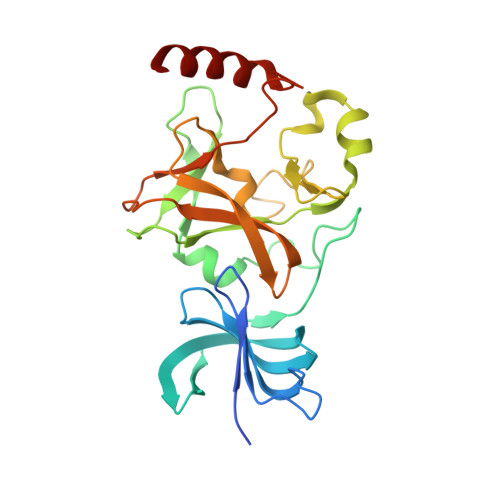
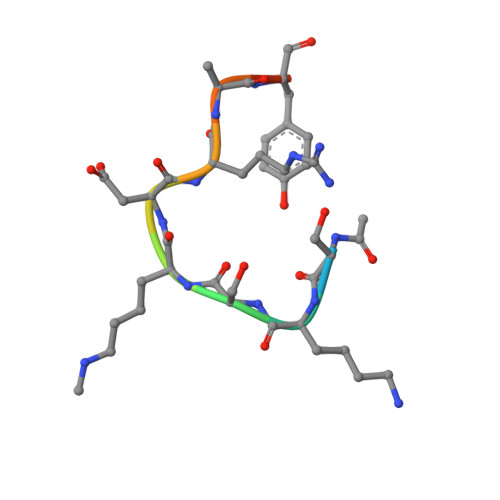
Structural basis for the methylation site specificity of SET7/9
Couture, J.-F., Collazo, E., Hauk, G., Trievel, R.C.(2006) Nat Struct Mol Biol 13: 140-146
- PubMed: 16415881
- DOI: https://doi.org/10.1038/nsmb1045
- Primary Citation of Related Structures:
2F69 - PubMed Abstract:
Human SET7/9 is a protein lysine methyltransferase (PKMT) that methylates histone H3, the tumor suppressor p53 and the TBP-associated factor TAF10. To elucidate the determinants of its substrate specificity, we have solved the enzyme's structure bound to a TAF10 peptide and examined its ability to methylate histone H3, TAF10 and p53 substrates bearing either mutations or covalent modifications within their respective methylation sites. Collectively, our data reveal that SET7/9 recognizes a conserved K/R-S/T/A motif preceding the lysine substrate and has a propensity to bind aspartates and asparagines on the C-terminal side of the lysine target. We then used a sequence-based approach with this motif to identify novel substrates for this PKMT. Among the putative targets is TAF7, which is methylated at Lys5 by the enzyme in vitro. These results demonstrate the predictive value of the consensus motif in identifying novel substrates for SET7/9.
- Department of Biological Chemistry, University of Michigan, 1301 Catherine Road, Ann Arbor, Michigan 48109-0606, USA.
Organizational Affiliation: