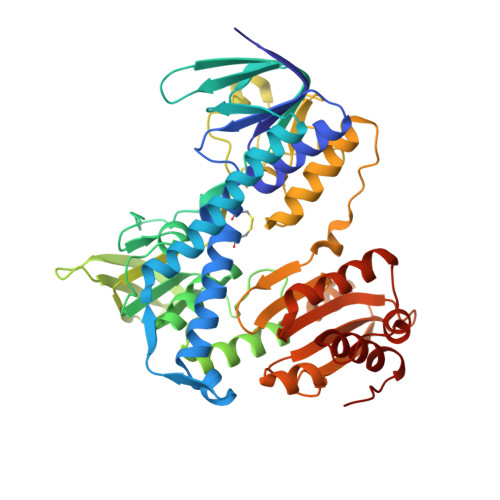
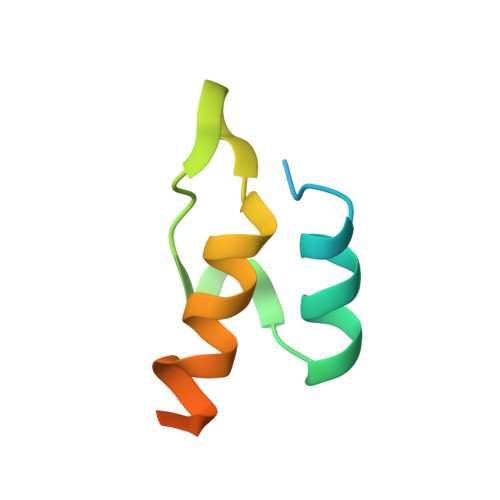
Structural Insight into Interactions between Dihydrolipoamide Dehydrogenase (E3) and E3 Binding Protein of Human Pyruvate Dehydrogenase Complex.
Brautigam, C.A., Wynn, R.M., Chuang, J.L., Machius, M., Tomchick, D.R., Chuang, D.T.(2006) Structure 14: 611-621
- PubMed: 16442803
- DOI: https://doi.org/10.1016/j.str.2006.01.001
- Primary Citation of Related Structures:
2F5Z, 2F60 - PubMed Abstract:
The 9.5 MDa human pyruvate dehydrogenase complex (PDC) utilizes the specific dihydrolipoamide dehydrogenase (E3) binding protein (E3BP) to tether the essential E3 component to the 60-meric core of the complex. Here, we report crystal structures of the binding domain (E3BD) of human E3BP alone and in complex with human E3 at 1.6 angstroms and 2.2 angstroms, respectively. The latter structure shows that residues from E3BD contact E3 across its 2-fold axis, resulting in one E3BD binding site on the E3 homodimer. Negligible conformational changes occur in E3BD upon its high-affinity binding to E3. Modifications of E3BD residues at the center of the E3BD/E3 interface impede E3 binding far more severely than those of residues on the periphery, validating the "hot spot" paradigm for protein interactions. A cluster of disease-causing E3 mutations located near the center of the E3BD/E3 interface prevents the efficient recruitment of these E3 variants by E3BP into the PDC, leading to the dysfunction of the PDC catalytic machine.
- Department of Biochemistry, The University of Texas Southwestern Medical Center at Dallas, Dallas, Texas 75390, USA. chad.brautigam@utsouthwestern.edu
Organizational Affiliation: