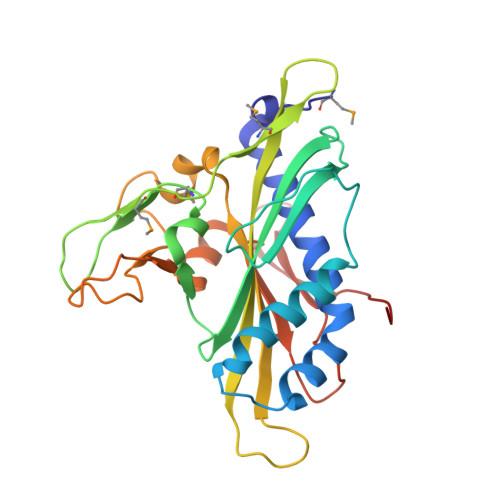
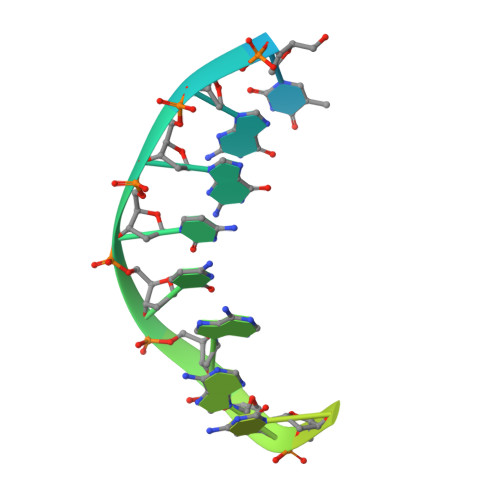
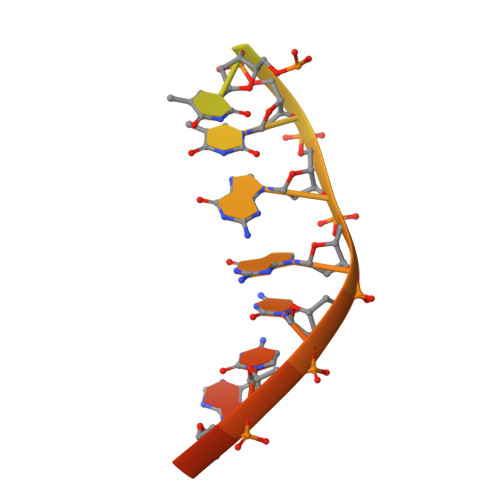
A view of consecutive binding events from structures of tetrameric endonuclease SfiI bound to DNA.
Vanamee, E.S., Viadiu, H., Kucera, R., Dorner, L., Picone, S., Schildkraut, I., Aggarwal, A.K.(2005) EMBO J 24: 4198-4208
- PubMed: 16308566
- DOI: https://doi.org/10.1038/sj.emboj.7600880
- Primary Citation of Related Structures:
2EZV, 2F03 - PubMed Abstract:
Many reactions in cells proceed via the sequestration of two DNA molecules in a synaptic complex. SfiI is a member of a growing family of restriction enzymes that can bind and cleave two DNA sites simultaneously. We present here the structures of tetrameric SfiI in complex with cognate DNA. The structures reveal two different binding states of SfiI: one with both DNA-binding sites fully occupied and the other with fully and partially occupied sites. These two states provide details on how SfiI recognizes and cleaves its target DNA sites, and gives insight into sequential binding events. The SfiI recognition sequence (GGCCNNNN[downward arrow]NGGCC) is a subset of the recognition sequence of BglI (GCCNNNN[downward arrow]NGGC), and both enzymes cleave their target DNAs to leave 3-base 3' overhangs. We show that even though SfiI is a tetramer and BglI is a dimer, and there is little sequence similarity between the two enzymes, their modes of DNA recognition are unusually similar.
- Structural Biology Program, Department of Physiology and Biophysics, Mount Sinai School of Medicine, New York, NY 10029, USA.
Organizational Affiliation: