Structural basis of the cytoplasmic tail of adhesion molecule CD43 and its binding to ERM proteins
Takai, Y., Kitano, K., Terawaki, S., Maesaki, R., Hakoshima, T.(2008) J Mol Biology 381: 634-644
- PubMed: 18614175
- DOI: https://doi.org/10.1016/j.jmb.2008.05.085
- Primary Citation of Related Structures:
2EMS - PubMed Abstract:
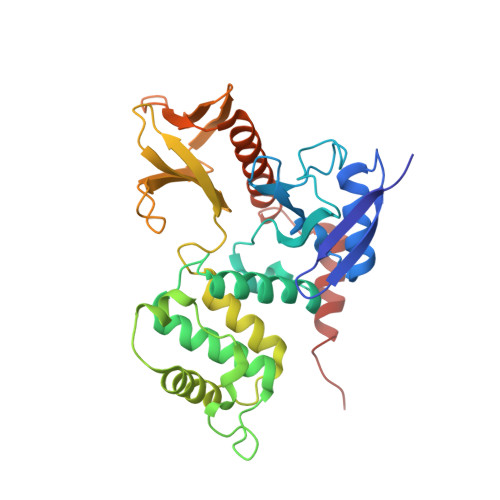
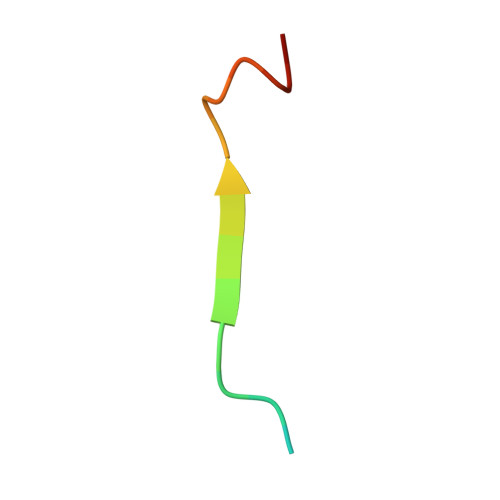
CD43/leukosialin/sialophorin is the major adhesion molecule in most hematopoietic cells and belongs to the sialomucin superfamily. In leukocyte emigration and activation, the exclusion of CD43 from the immunological synapse is an essential step. While the exclusion requires binding of the cytoplasmic region to ERM (ezrin/radixin/moesin) proteins, the detailed specific nature of the interaction between CD43 and ERM proteins is obscure. We have characterized the conformational properties of the CD43 cytoplasmic region, consisting of 124 amino acid residues, by hydrodynamic and spectroscopic measurements. Sedimentation equilibrium and velocity studies of ultracentrifugation revealed that the CD43 cytoplasmic peptide exists in a monomeric and extended form in solution. The crystal structure of the complex between the radixin FERM (4.1 and ERM) domain and the CD43 juxtamembrane region peptide reveals that the nonpolar region of the peptide binds subdomain C of the FERM domain. CD43 lacks the Motif-1 sequence for FERM binding found in the FERM-intercellular adhesion molecule-2 complex but possesses two conserved leucine residues that dock into the hydrophobic pocket of subdomain C without forming a 3(10)-helix. The FERM-binding site on CD43 is overlapped with the functional nuclear localization signal sequence. Our structure suggests that regulation of ERM binding may be coupled with regulated intramembrane proteolysis of CD43 followed by the nuclear transfer of the cytoplasmic peptide.
- Structural Biology Laboratory, Nara Institute of Science and Technology, 8916-5 Takayama, Ikoma, Nara 630-0192, Japan.
Organizational Affiliation: