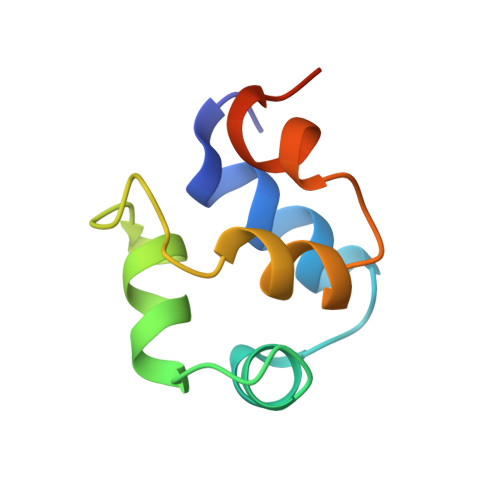
Structure of phage 434 Cro protein at 2.35 A resolution.
Mondragon, A., Wolberger, C., Harrison, S.C.(1989) J Mol Biology 205: 179-188
- PubMed: 2647998
- DOI: https://doi.org/10.1016/0022-2836(89)90374-4
- Primary Citation of Related Structures:
2CRO - PubMed Abstract:
The crystal structure of phage 434 Cro protein has been determined and refined against 2.35 A data to an R-factor of 19.5%. The protein comprises five alpha-helices and shows the helix-turn-helix motif found in other repressor proteins.
- Department of Biochemistry and Molecular Biology, Harvard University, Cambridge, MA.
Organizational Affiliation: