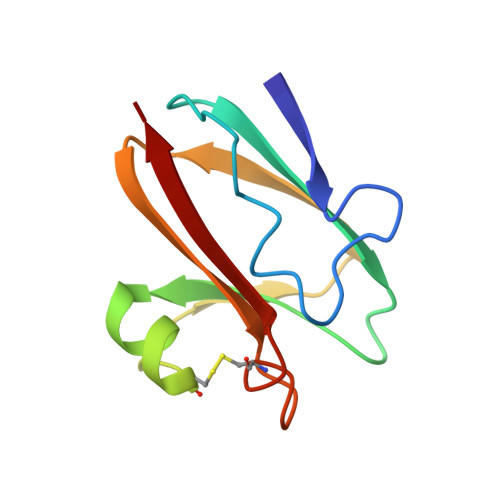
The structure of a phytocyanin, the basic blue protein from cucumber, refined at 1.8 A resolution.
Guss, J.M., Merritt, E.A., Phizackerley, R.P., Freeman, H.C.(1996) J Mol Biology 262: 686-705
- PubMed: 8876647
- DOI: https://doi.org/10.1006/jmbi.1996.0545
- Primary Citation of Related Structures:
2CBP - PubMed Abstract:
The crystal structure of the cucumber basic protein (CBP), a type 1 or blue copper protein, has been refined at 1.8 A resolution. The molecule resembles other blue copper proteins in having a Greek key beta-barrel structure, except that the barrel is open on one side and is better described as a "beta-sandwich" or "beta-taco". The Cu atom has the normal blue copper NNSS' co-ordination with bond lengths Cu-N(His39) = 1.93 A, Cu-S(Cys79) = 2.16 A, Cu-N(His84) = 1.95 A, Cu-S(Met89) = 2.61 A. The Cu-S(Met) bond is the shortest so far observed in a blue copper protein. A disulphide link, (Cys52)-S-S-(Cys85), appears to play an important role in stabilising the molecular structure. It is suggested that the polypeptide fold is typical of a sub-family of blue copper proteins (phytocyanins) as well as a non-metalloprotein, ragweed allergen Ra3, with which CBP has a high degree of sequence identify. The proteins currently identifiable as phytocyanins are CBP, stellacyanin, mavicyanin, umecyanin, a cucumber peeling cupredoxin, a putative blue copper protein in pea pods, and a blue copper protein from Arabidopsis thaliana. In all except CBP and the pea-pod protein, the axial methionine ligand normally found at blue copper sites is replaced by glutamine. The structure of CBP was originally solved by the multiple wavelength anomalous scattering method, using data recorded at four wavelengths. All these data were included in the restrained least squares refinement. The final model comprises 96 amino acid residues, 122 solvent molecules and a copper atom. Several residues are modelled as having more than one conformation. The residual R is 0.141 for 41,910 observations (including Bijvoet-related observations) of 8.142 unique reflections in the resolution range 7 to 1.8 A.
- School of Chemistry, University of Sydney, NSW, Australia.
Organizational Affiliation: