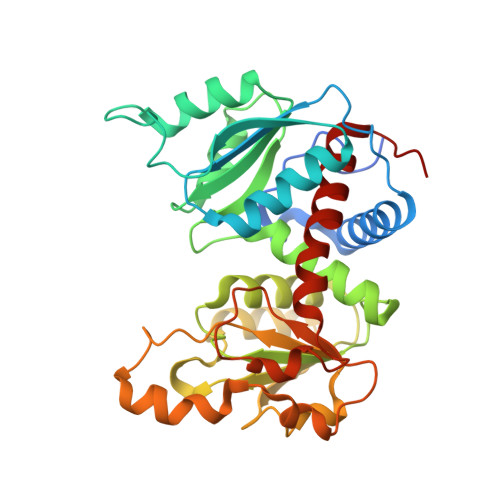
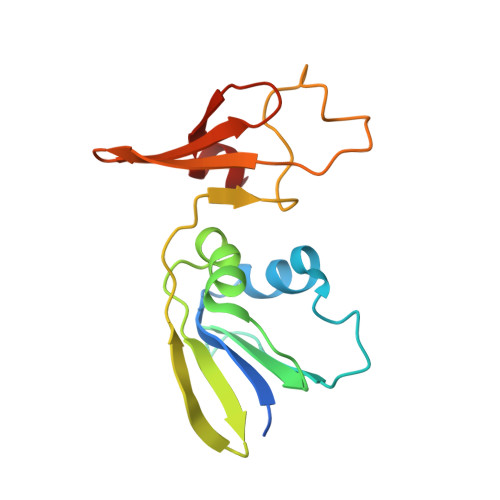
Structural investigation of cold activity and regulation of aspartate carbamoyltransferase from the extreme psychrophilic bacterium Moritella profunda.
De Vos, D., Xu, Y., Hulpiau, P., Vergauwen, B., Van Beeumen, J.J.(2007) J Mol Biology 365: 379-395
- PubMed: 17070547
- DOI: https://doi.org/10.1016/j.jmb.2006.09.064
- Primary Citation of Related Structures:
2BE7 - PubMed Abstract:
Aspartate carbamoyltransferase (EC 2.1.3.2) is extensively studied as a model for cooperativity and allosteric regulation. The structure of the Escherichia coli enzyme has been thoroughly analyzed by X-ray crystallography, and recently the crystal structures of two hyperthermophilic ATCases of the same structural class have been characterized. We here report the detailed functional and structural investigation of the ATCase from the psychrophilic deep sea bacterium Moritella profunda. Our analysis indicates that the enzyme conforms to the E. coli model in that two allosteric states exist that are influenced by similar homotropic interactions. The heterotropic properties differ in that CTP and UTP inhibit the holoenzyme, but ATP seems to exhibit a dual regulatory pattern, activating the enzyme at low concentrations and inhibiting it in the mM range. The crystal structure of the unliganded M. profunda ATCase shows resemblance to a more extreme T state reported previously for an E. coli ATCase mutant. A detailed molecular analysis reveals potential features of adaptation to cold activity and cold regulation. Moreover, M. profunda ATCase presents similarities with certain mutants of E. coli ATCase altered in their kinetic properties or temperature relationships. Finally, structural and functional comparison of ATCases across the full physiological temperature range agrees with an important, but fundamentally different role for electrostatics in protein adaptation at both extremes, i.e. an increased stability through the formation of ion pairs and ion pair networks at high physiological temperatures, and an increased flexibility through enhanced protein solvation at low temperatures.
- Laboratory of Protein Biochemistry and Protein Engineering, Ghent University, K.L. Ledeganckstraat 35, 9000 Gent, Belgium.
Organizational Affiliation: