The high-resolution crystal structure of an allergenic thaumatin-like protein, Pru av 2, isolated from ripe cherries
Dall'Antonia, Y., Pavkov, T., Fuchs, H., Breiteneder, H., Keller, W.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Thaumatin-like protein | 222 | Prunus avium | Mutation(s): 0 EC: 3.2.1.39 | 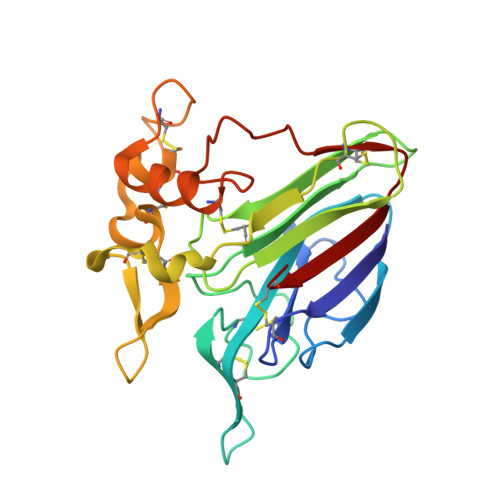 | |
UniProt | |||||
Find proteins for P50694 (Prunus avium) Explore P50694 Go to UniProtKB: P50694 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P50694 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 44.288 | α = 90 |
| b = 41.15 | β = 107.08 |
| c = 58.946 | γ = 90 |
| Software Name | Purpose |
|---|---|
| DENZO | data reduction |
| SCALEPACK | data scaling |
| MOLREP | phasing |
| SHELX | refinement |
| PDB_EXTRACT | data extraction |
| SHELXL-97 | refinement |