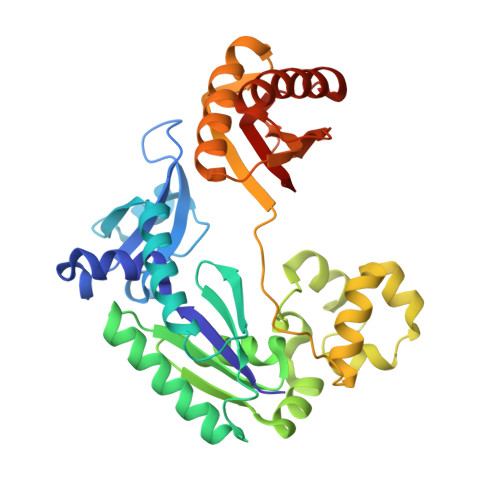
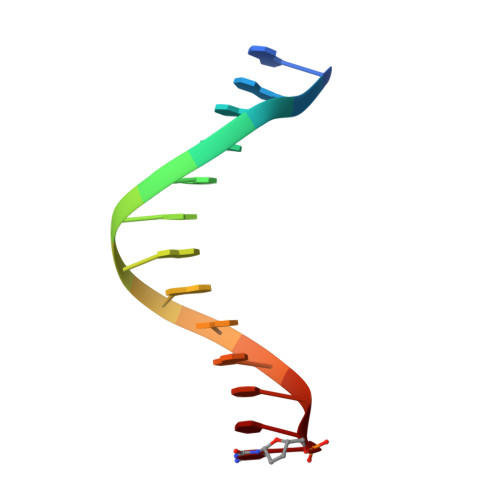
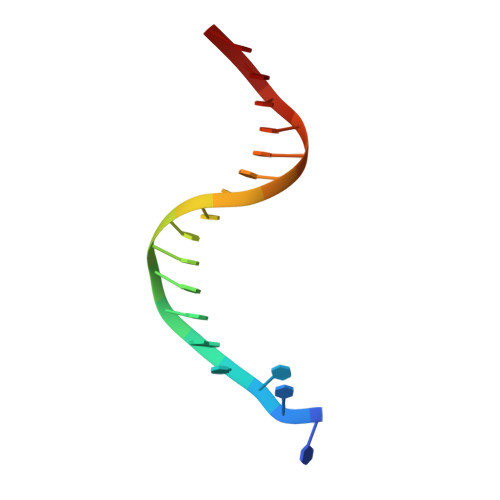
Fidelity of Dpo4: effect of metal ions, nucleotide selection and pyrophosphorolysis.
Vaisman, A., Ling, H., Woodgate, R., Yang, W.(2005) EMBO J 24: 2957-2967
- PubMed: 16107880
- DOI: https://doi.org/10.1038/sj.emboj.7600786
- Primary Citation of Related Structures:
2AGO, 2AGP, 2AGQ - PubMed Abstract:
We report the crystal structures of a translesion DNA polymerase, Dpo4, complexed with a matched or mismatched incoming nucleotide and with a pyrophosphate product after misincorporation. These structures suggest two mechanisms by which Dpo4 may reject a wrong incoming nucleotide with its preformed and open active site. First, a mismatched replicating base pair leads to poor base stacking and alignment of the metal ions and as a consequence, inhibits incorporation. By replacing Mg2+ with Mn2+, which has a relaxed coordination requirement and tolerates misalignment, the catalytic efficiency of misincorporation increases dramatically. Mn2+ also enhances translesion synthesis by Dpo4. Subtle conformational changes that lead to the proper metal ion coordination may, therefore, be a key step in catalysis. Second, the slow release of pyrophosphate may increase the fidelity of Dpo4 by stalling mispaired primer extension and promoting pyrophosphorolysis that reverses the polymerization reaction. Indeed, Dpo4 has robust pyrophosphorolysis activity and degrades the primer strand in the presence of pyrophosphate. The correct incoming nucleotide allows DNA synthesis to overcome pyrophosphorolysis, but an incorrect incoming nucleotide does not.
- Laboratory of Genomic Integrity, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD 20892, USA.
Organizational Affiliation: