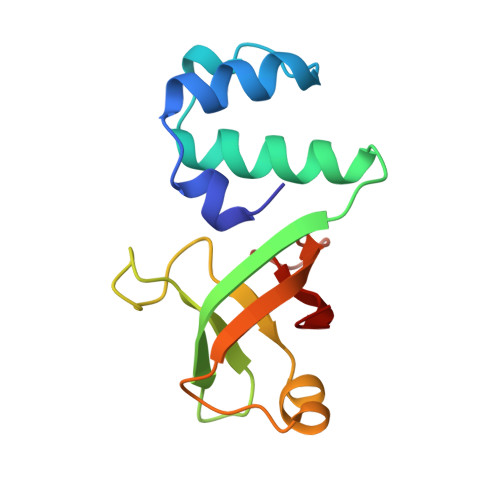
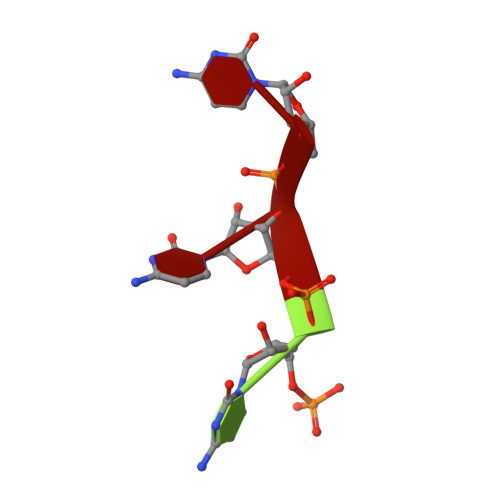
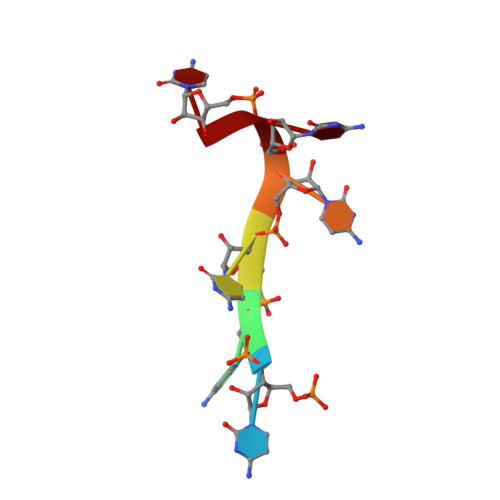
The structural basis for terminator recognition by the Rho transcription termination factor.
Bogden, C.E., Fass, D., Bergman, N., Nichols, M.D., Berger, J.M.(1999) Mol Cell 3: 487-493
- PubMed: 10230401
- DOI: https://doi.org/10.1016/s1097-2765(00)80476-1
- Primary Citation of Related Structures:
1A8V, 2A8V - PubMed Abstract:
The E. coli Rho protein disengages newly transcribed RNA from its DNA template, helping terminate certain transcripts. We have determined the X-ray crystal structure of the RNA-binding domain of Rho complexed to an RNA ligand. Filters that screen both ligand size and chemical functionality line the primary nucleic acid-binding site, imparting sequence specificity to a generic single-stranded nucleic acid-binding fold and explaining the preference of Rho for cytosine-rich RNA. The crystal packing reveals two Rho domain protomers bound to a single RNA with a single base spacer, suggesting that the strong RNA-binding sites of Rho may arise from pairing of RNA-binding modules. Dimerization of symmetric subunits on an asymmetric ligand is developed as a model for allosteric control in the action of the intact Rho hexamer.
- Whitehead Institute for Biomedical Research, 9 Cambridge Center, Massachusetts 02142, USA.
Organizational Affiliation: