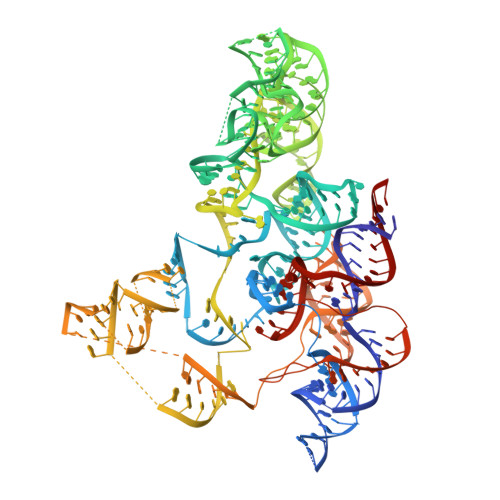
Crystal structure of the RNA component of bacterial ribonuclease P.
Torres-Larios, A., Swinger, K.K., Krasilnikov, A.S., Pan, T., Mondragon, A.(2005) Nature 437: 584-587
- PubMed: 16113684
- DOI: https://doi.org/10.1038/nature04074
- Primary Citation of Related Structures:
2A2E - PubMed Abstract:
Transfer RNA (tRNA) is produced as a precursor molecule that needs to be processed at its 3' and 5' ends. Ribonuclease P is the sole endonuclease responsible for processing the 5' end of tRNA by cleaving the precursor and leading to tRNA maturation. It was one of the first catalytic RNA molecules identified and consists of a single RNA component in all organisms and only one protein component in bacteria. It is a true multi-turnover ribozyme and one of only two ribozymes (the other being the ribosome) that are conserved in all kingdoms of life. Here we show the crystal structure at 3.85 A resolution of the RNA component of Thermotoga maritima ribonuclease P. The entire RNA catalytic component is revealed, as well as the arrangement of the two structural domains. The structure shows the general architecture of the RNA molecule, the inter- and intra-domain interactions, the location of the universally conserved regions, the regions involved in pre-tRNA recognition and the location of the active site. A model with bound tRNA is in agreement with all existing data and suggests the general basis for RNA-RNA recognition by this ribozyme.
- Department of Biochemistry, Molecular Biology and Cell Biology, Northwestern University, Evanston, Illinois 60208, USA.
Organizational Affiliation: