Crystal structure of Phosphoribosyl-ATP pyrophosphatase from Streptomyces coelicolor. NESG target RR8.
Benach, J., Kuzin, A.P., Forouhar, F., Abashidze, M., Vorobiev, S.M., Rong, X., Acton, T.B., Montelione, G.T., Hunt, J.F.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Phosphoribosyl-ATP pyrophosphatase | 98 | Streptomyces coelicolor | Mutation(s): 4 Gene Names: hisE EC: 3.6.1.31 | 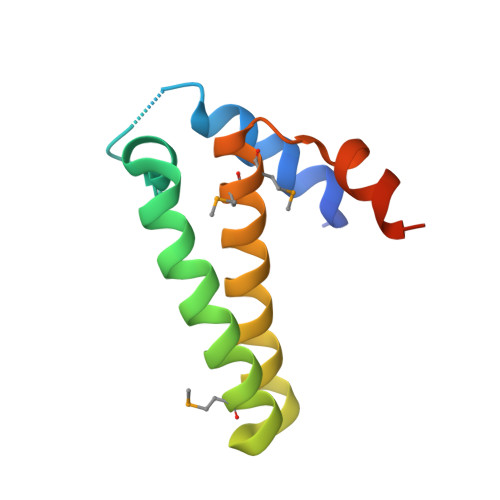 | |
UniProt | |||||
Find proteins for Q9EWK0 (Streptomyces coelicolor (strain ATCC BAA-471 / A3(2) / M145)) Explore Q9EWK0 Go to UniProtKB: Q9EWK0 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9EWK0 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| MSE Query on MSE | A, B, C, D, E A, B, C, D, E, F, G, H | L-PEPTIDE LINKING | C5 H11 N O2 Se |  | MET |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 44.904 | α = 79.21 |
| b = 62.361 | β = 82.13 |
| c = 76.62 | γ = 75.42 |
| Software Name | Purpose |
|---|---|
| CNS | refinement |
| HKL-2000 | data reduction |
| SCALEPACK | data scaling |
| SOLVE | phasing |