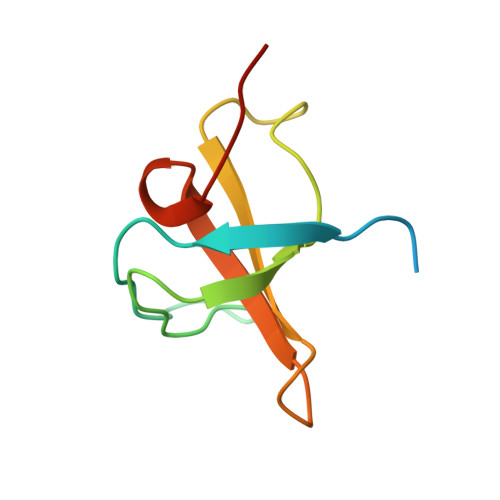
Regulation of RhoGEF Activity by Intramolecular and Intermolecular SH3 Domain Interactions.
Schiller, M.R., Chakrabarti, K., King, G.F., Schiller, N.I., Eipper, B.A., Maciejewski, M.W.(2006) J Biological Chem 281: 18774-18786
- PubMed: 16644733
- DOI: https://doi.org/10.1074/jbc.M512482200
- Primary Citation of Related Structures:
1U3O - PubMed Abstract:
RhoGEFs are central controllers of small G-proteins in cells and are regulated by several mechanisms. There are at least 22 human RhoGEFs that contain SH3 domains, raising the possibility that, like several other enzymes, SH3 domains control the enzymatic activity of guanine nucleotide exchange factor (GEF) domains through intra- and/or intermolecular interactions. The structure of the N-terminal SH3 domain of Kalirin was solved using NMR spectroscopy, and it folds much like other SH3 domains. However, NMR chemical shift mapping experiments showed that this Kalirin SH3 domain is unique, containing novel cooperative binding site(s) for intramolecular PXXP ligands. Intramolecular Kalirin SH3 domain/ligand interactions, as well as binding of the Kalirin SH3 domain to the adaptor protein Crk, inhibit the GEF activity of Kalirin. This study establishes a novel molecular mechanism whereby intramolecular and intermolecular Kalirin SH3 domain/ligand interactions modulate GEF activity, a regulatory mechanism that is likely used by other RhoGEF family members.
- Department of Neuroscience, University of Connecticut Health Center, Farmington, Connecticut 06019-4301, USA. schiller@nso.uchc.edu
Organizational Affiliation: