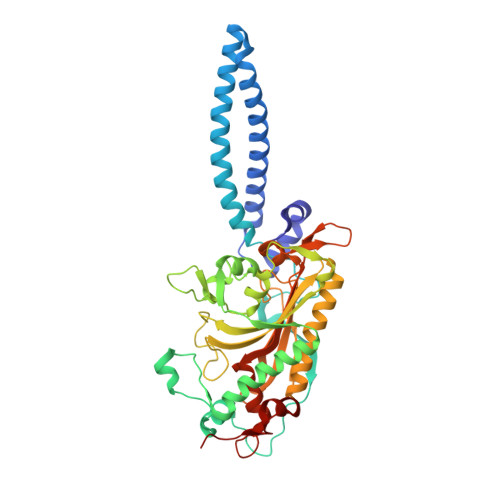
Crystal structures at 2.5 angstrom resolution of seryl-tRNA synthetase complexed with two analogs of seryl adenylate.
Belrhali, H., Yaremchuk, A., Tukalo, M., Larsen, K., Berthet-Colominas, C., Leberman, R., Beijer, B., Sproat, B., Als-Nielsen, J., Grubel, G., Legrand, J.-F., Lehmann, M., Cusack, S.(1994) Science 263: 1432-1436
- PubMed: 8128224
- DOI: https://doi.org/10.1126/science.8128224
- Primary Citation of Related Structures:
1SES, 1SET - PubMed Abstract:
Crystal structures of seryl-tRNA synthetase from Thermus thermophilus complexed with two different analogs of seryl adenylate have been determined at 2.5 A resolution. The first complex is between the enzyme and seryl-hydroxamate-AMP (adenosine monophosphate), produced enzymatically in the crystal from adenosine triphosphate (ATP) and serine hydroxamate, and the second is with a synthetic analog of seryl adenylate (5'-O-[N-(L-seryl)-sulfamoyl]adenosine), which is a strong inhibitor of the enzyme. Both molecules are bound in a similar fashion by a network of hydrogen bond interactions in a deep hydrophilic cleft formed by the antiparallel beta sheet and surrounding loops of the synthetase catalytic domain. Four regions in the primary sequence are involved in the interactions, including the motif 2 and 3 regions of class 2 synthetases. Apart from the specific recognition of the serine side chain, the interactions are likely to be similar in all class 2 synthetases.
- EMBL Grenoble Outstation, France.
Organizational Affiliation: