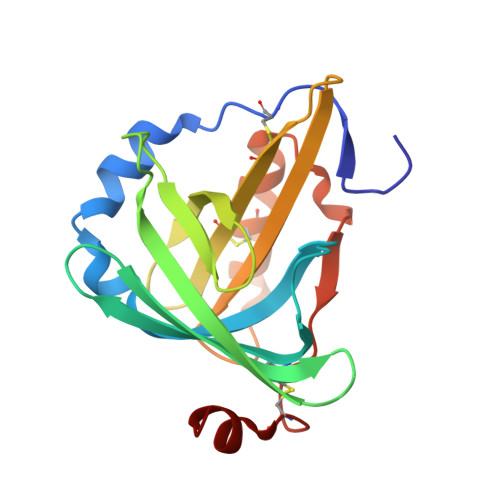
The structure and refinement of apocrustacyanin C2 to 1.3 A resolution and the search for differences between this protein and the homologous apoproteins A1 and C1.
Habash, J., Helliwell, J.R., Raftery, J., Cianci, M., Rizkallah, P.J., Chayen, N.E., Nneji, G.A., Zagalsky, P.F.(2004) Acta Crystallogr D Biol Crystallogr 60: 493-498
- PubMed: 14993674
- DOI: https://doi.org/10.1107/S090744490400037X
- Primary Citation of Related Structures:
1S2P, 1S44 - PubMed Abstract:
The blue carotenoprotein alpha-crustacyanin of Homarus gammarus lobster carapace is comprised chemically of five 20 kDa subunits. Only two genes for the proteins have been isolated (J. B. C. Findlay, personal communication) and the five apoproteins fall into two sets of homologous proteins based on their chemical properties (CRTC, consisting of apoproteins C(1), C(2) and A(1), and CRTA, consisting of apoproteins A(2) and A(3)). The diffraction quality of apo C(2) has been improved from 2.2 to 1.3 A and its structure solved. The structure is compared with the A(1) and C(1) proteins determined at 1.4 A [Cianci et al. (2001), Acta Cryst. D57, 1219-1229] and 1.15 A, respectively [Gordon et al. (2001), Acta Cryst. D57, 1230-1237] and found to be very similar. Normalized B-factor difference plots per residue of different types were used to try to find chemically modified residues; none were found at these resolutions. It remains possible that the differences between the CRTC proteins result from differences in amidation. By comparison of a crystal grown with glycerol (studied at 1.6 A) and one grown without glycerol (studied at 1.3 A) it was seen that glycerol bound at the astaxanthin site.
- Section of Structural Chemistry, Department of Chemistry, University of Manchester, Manchester M13 9PL, England.
Organizational Affiliation: