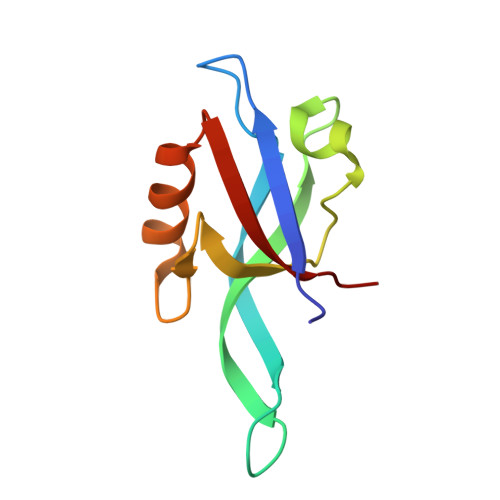
Cdc42 regulates the Par-6 PDZ domain through an allosteric CRIB-PDZ transition.
Peterson, F.C., Penkert, R.R., Volkman, B.F., Prehoda, K.E.(2004) Mol Cell 13: 665-676
- PubMed: 15023337
- DOI: https://doi.org/10.1016/s1097-2765(04)00086-3
- Primary Citation of Related Structures:
1RY4, 1RZX - PubMed Abstract:
Regulation of protein interaction domains is required for cellular signaling dynamics. Here, we show that the PDZ protein interaction domain from the cell polarity protein Par-6 is regulated by the Rho GTPase Cdc42. Cdc42 binds to a CRIB domain adjacent to the PDZ domain, increasing the affinity of the Par-6 PDZ for its carboxy-terminal ligand by approximately 13-fold. Par-6 PDZ regulation is required for function as mutational disruption of Cdc42-Par-6 PDZ coupling leads to inactivation of Par-6 in polarized MDCK epithelial cells. Structural analysis reveals that the free PDZ domain has several deviations from the canonical PDZ conformation that account for its low ligand affinity. Regulation results from a Cdc42-induced conformational transition in the CRIB-PDZ module that causes the PDZ to assume a canonical, high-affinity PDZ conformation. The coupled CRIB and PDZ architecture of Par-6 reveals how simple binding domains can be combined to yield complex regulation.
- Department of Biochemistry, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226 USA.
Organizational Affiliation: