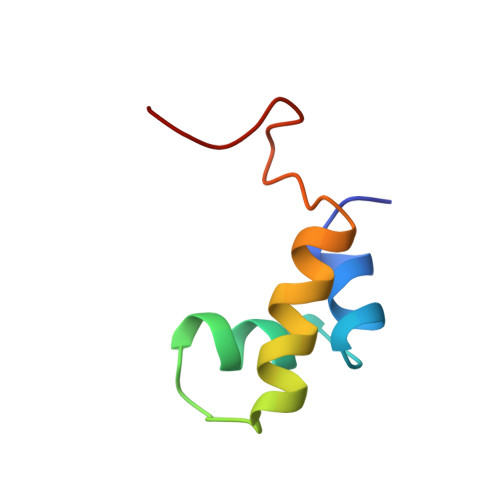
Structural comparison of the free and DNA-bound forms of the purine repressor DNA-binding domain.
Nagadoi, A., Morikawa, S., Nakamura, H., Enari, M., Kobayashi, K., Yamamoto, H., Sampei, G., Mizobuchi, K., Schumacher, M.A., Brennan, R.G., Nishimura, Y.(1995) Structure 3: 1217-1224
- PubMed: 8591032
- DOI: https://doi.org/10.1016/s0969-2126(01)00257-x
- Primary Citation of Related Structures:
1PRU, 1PRV - PubMed Abstract:
The purine repressor (PurR) regulates genes that encode enzymes for purine biosynthesis. PurR has a two domain structure with an N-terminal DNA-binding domain (DBD) and a C-terminal corepressor-binding domain (CBD). The three dimensional structure of a ternary complex of PurR bound to both corepressor and a specific DNA sequence has recently been determined by X-ray crystallography. We have determined the solution structure of the PurR DBD by NMR. It contains three helices, with the first and second helices forming a helix-turn-helix motif. The tertiary structure of the three helices is very similar to that of the corresponding region in the ternary complex. The structure of the hinge helical region, however, which makes specific base contacts in the minor groove of DNA, is disordered in the DNA-free form. The stable formation of PurR hinge helices requires PurR dimerization, which brings the hinge regions proximal to each other. The dimerization of the hinge helices is likely to be controlled by the CBD dimerization interface, but is induced by specific-DNA binding.
- Graduate School of Integrated Science, Yokohama City University, Japan.
Organizational Affiliation: