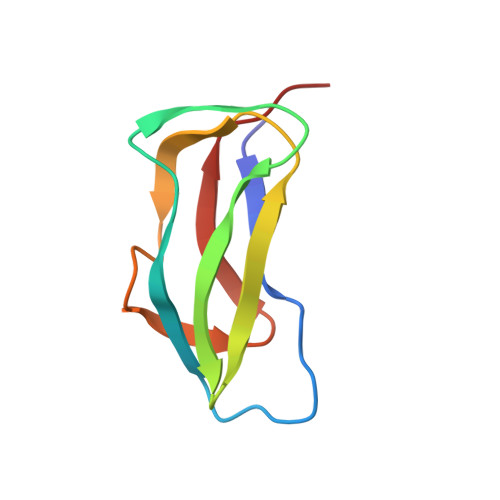
Three-dimensional structure of the lipoyl domain from the dihydrolipoyl succinyltransferase component of the 2-oxoglutarate dehydrogenase multienzyme complex of Escherichia coli.
Ricaud, P.M., Howard, M.J., Roberts, E.L., Broadhurst, R.W., Perham, R.N.(1996) J Mol Biology 264: 179-190
- PubMed: 8950276
- DOI: https://doi.org/10.1006/jmbi.1996.0632
- Primary Citation of Related Structures:
1PMR - PubMed Abstract:
A sub-gene encoding the lipoyl domain of the dihydrolipoyl succinyltransferase polypeptide chain of the 2-oxoglutarate dehydrogenase multienzyme complex of Escherichia coli was over-expressed and the protein was purified uniformly labelled with 15N. The three-dimensional structure of the domain was determined by means of nuclear magnetic resonance spectroscopy, based on 905 nuclear Overhauser effect inter-proton distance restraints, 42 phi torsion angle restraints and hydrogen bond restraints from 24 slowly exchanging amide protons. The structure of the 80-residue domain is that of a flattened beta-barrel surrounding a hydrophobic core in which Trp22 plays a central role in anchoring two four-stranded sheets together. The polypeptide backbone exhibits a 2-fold axis of quasi-symmetry, with the lipoylation site, Lys43, located at the tip of an exposed beta-turn in one beta-sheet and the N and C-terminal residues close together in space in the other beta-sheet. The atomic r.m.s. distribution about the mean coordinate is 0.46 A for the backbone atoms in the highly structured region and 0.88 A along the entire backbone (residues Ser1 to Asn80), including a less well-defined surface loop and the lipoyl-lysine beta-turn. The structure closely resembles that of the lipoyl domains from pyruvate dehydrogenase complexes, in accord with the existence of strongly conserved residues at critical positions in the domains. The structures of the lipoyl domains throw light on the requirements for the specificity of reductive acylation of their pendant lipoyl groups in the parent 2-oxo acid dehydrogenase complexes; an important aspect of the mechanisms underlying active site coupling and substrate channelling.
- Cambridge Centre for Molecular Recognition, Department of Biochemistry, University of Cambridge, UK.
Organizational Affiliation: