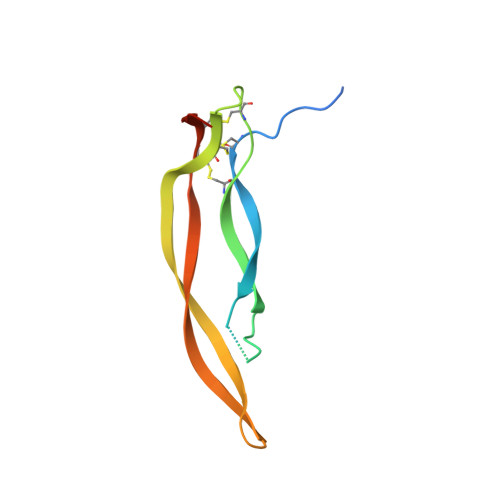
Crystal structure of human platelet-derived growth factor BB.
Oefner, C., D'Arcy, A., Winkler, F.K., Eggimann, B., Hosang, M.(1992) EMBO J 11: 3921-3926
- PubMed: 1396586
- DOI: https://doi.org/10.1002/j.1460-2075.1992.tb05485.x
- Primary Citation of Related Structures:
1PDG - PubMed Abstract:
The crystal structure of the homodimeric BB isoform of human recombinant platelet-derived growth factor (PDGF-BB) has been determined by X-ray analysis to 3.0 A resolution. The polypeptide chain is folded into two highly twisted antiparallel pairs of beta-strands and contains an unusual knotted arrangement of three intramolecular disulfide bonds. Dimerization leads to the clustering of three surface loops at each end of the elongated dimer, which most probably form the receptor recognition sites.
- Department of Pharmaceutical Research--New Technologies, F. Hoffmann-La Roche Ltd., Basel, Switzerland.
Organizational Affiliation: