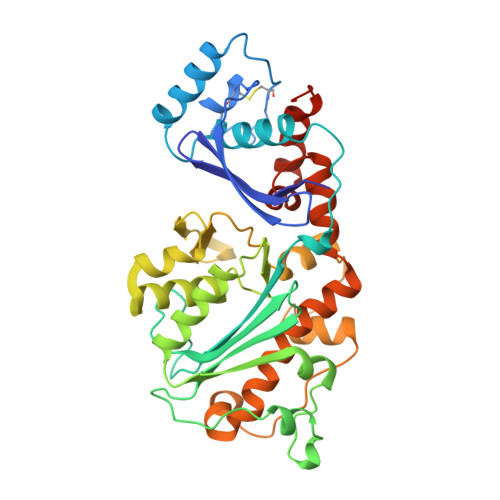
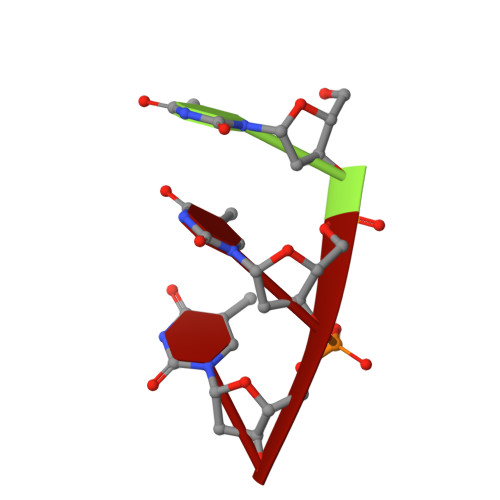
Crystal structures of an NH2-terminal fragment of T4 DNA polymerase and its complexes with single-stranded DNA and with divalent metal ions.
Wang, J., Yu, P., Lin, T.C., Konigsberg, W.H., Steitz, T.A.(1996) Biochemistry 35: 8110-8119
- PubMed: 8679562
- DOI: https://doi.org/10.1021/bi960178r
- Primary Citation of Related Structures:
1NOY, 1NOZ - PubMed Abstract:
We report the crystal structure of an NH2-terminal 388-residue fragment of T4 DNA polymerase (protein N388) refined at 2.2 A resolution. This fragment contains both the 3'-5' exonuclease active site and part of the autologous mRNA binding site (J. D. Karam, personal communication). The structure of a complex between the apoprotein N388 and a substrate, p(dT)3, has been refined at 2.5 A resolution to a crystallographic R-factor of 18.7%. Two divalent metal ion cofactors, Zn(II) and Mn(II), have been located in crystals of protein N388 which had been soaked in solutions containing Zn(II), Mn(II), or both. The structure of the 3'-5' exonuclease domain of protein N388 closely resembles the corresponding region in the Klenow fragment despite minimal sequence identity. The side chains of four carboxylate residues that serve as ligands for the two metal ions required for catalysis are located in geometrically equivalent positions in both proteins with a rms deviation of 0.87 A. There are two main differences between the 3'-5' exonuclease active site regions of the two proteins: (I) the OH of Tyr-497 in the Klenow fragment interacts with the scissile phosphate in the active site whereas the OH of the equivalent tyrosine (Tyr-320) in protein N388 points away from the active center; (II) different residues form of the binding pocket for the 3'-terminal bases of the substrate. In the protein N388 complex the 3'-terminal base of p(dT)3 is rotated approximately 60 degrees relative to the position that the corresponding base occupies in the p(dT)3 complex with the Klenow fragment. Finally, a separate domain (residues 1-96) of protein N388 may be involved in mRNA binding that results in translational regulation of T4 DNA polymerase (Pavlov & Karam, 1994).
- Department of Molecular Biophysics and Biochemistry Howard Hughes Medical Institute, Yale University, New Haven, Connecticut 06520-8114, USA.
Organizational Affiliation: