X-ray structure of the human hyperplastic discs protein: an ortholog of the C-terminal domain of poly(A)-binding protein.
Deo, R.C., Sonenberg, N., Burley, S.K.(2001) Proc Natl Acad Sci U S A 98: 4414-4419
- PubMed: 11287654
- DOI: https://doi.org/10.1073/pnas.071552198
- Primary Citation of Related Structures:
1I2T - PubMed Abstract:
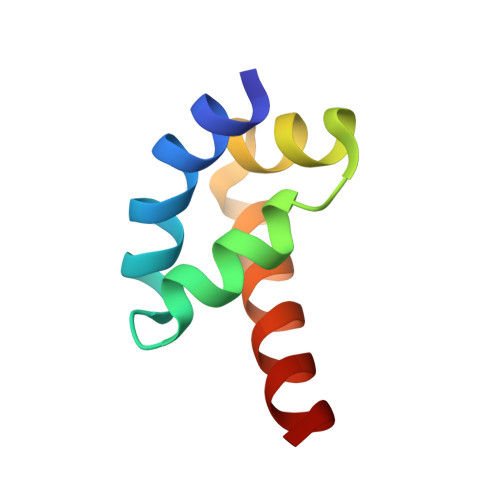
The poly(A)-binding protein (PABP) recognizes the 3' mRNA poly(A) tail and plays an essential role in eukaryotic translation initiation and mRNA stabilization/degradation. PABP is a modular protein, with four N-terminal RNA-binding domains and an extensive C terminus. The C-terminal region of PABP is essential for normal growth in yeast and has been implicated in mediating PABP homo-oligomerization and protein-protein interactions. A small, proteolytically stable, highly conserved domain has been identified within this C-terminal segment. Remarkably, this domain is also present in the hyperplastic discs protein (HYD) family of ubiquitin ligases. To better understand the function of this conserved region, an x-ray structure of the PABP-like segment of the human HYD protein has been determined at 1.04-A resolution. The conserved domain adopts a novel fold resembling a right-handed supercoil of four alpha-helices. Sequence profile searches and comparative protein structure modeling identified a small ORF from the Arabidopsis thaliana genome that encodes a structurally similar but distantly related PABP/HYD domain. Phylogenetic analysis of the experimentally determined (HYD) and homology modeled (PABP) protein surfaces revealed a conserved feature that may be responsible for binding to a PABP interacting protein, Paip1, and other shared interaction partners.
- Laboratories of Molecular Biophysics, Howard Hughes Medical Institute, The Rockefeller University, 1230 York Avenue, New York, NY 10021, USA.
Organizational Affiliation: