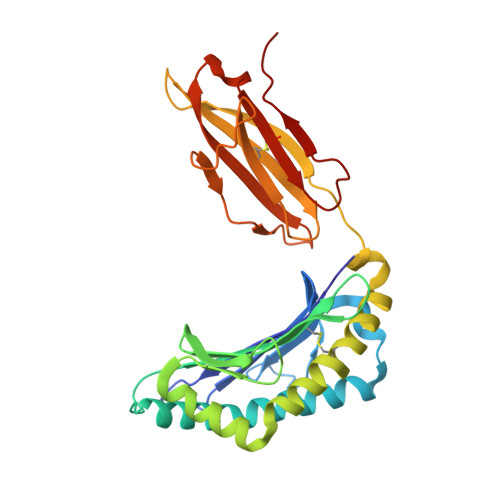
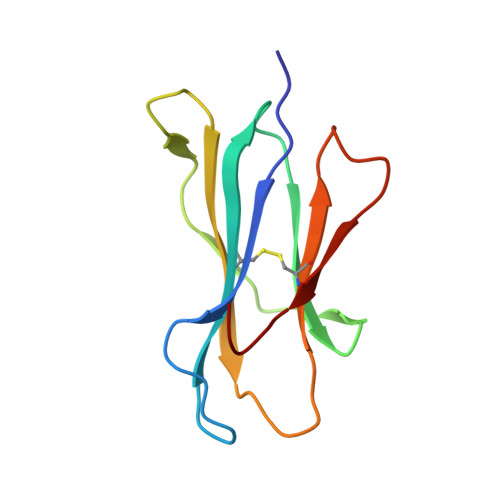
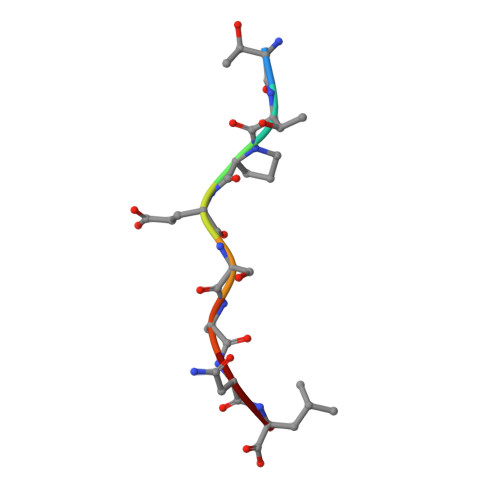
First glimpse of the peptide presentation by rhesus macaque MHC class I: crystal structures of Mamu-A*01 complexed with two immunogenic SIV epitopes and insights into CTL escape.
Chu, F., Lou, Z., Chen, Y.W., Liu, Y., Gao, B., Zong, L., Khan, A.H., Bell, J.I., Rao, Z., Gao, G.F.(2007) J Immunol 178: 944-952
- PubMed: 17202356
- DOI: https://doi.org/10.4049/jimmunol.178.2.944
- Primary Citation of Related Structures:
1ZVS - PubMed Abstract:
The infection of rhesus macaques (Macaca mulatta) by the SIV is the best animal model for studying HIV infection and for AIDS vaccine development. A prevalent MHC class I allele, Mamu-A*01, is known to correlate with containment of SIV, which has been extensively explored in studies of CTL-based vaccination concepts. We determined the crystal structures of Mamu-A*01 complexed with two immunodominant SIV epitopes: the nonamer CM9 of group-specific Ag (Gag, 181-189; CTPYDINQM) and the octamer TL8 of transcription activator (Tat, 28-35; TTPESANL). The overall structures of the two Mamu-A*01 complexes are similar to other MHC class I molecules. Both structures confirm the presence of an absolutely conserved proline anchor residue in the P3 position of the Ag, bound to a D pocket of the Mamu-A*01 H chain with optimal surface complementarity. Like other MHC/peptide complex structures, the P2 and C-terminal residues of the epitopes are also important for anchoring to the MHC molecule, whereas the middle residues form an arch and their side chains are directed into solvent. These two structures reveal details of how Mamu-A*01 interacts with two well-studied epitopes at the atomic level. We discuss the structural basis of CTL escape, based on molecular models made possible by these two structures. The results we present in this study are most relevant for the rational design of Mamu-A*01-restricted CTL epitopes with improved binding, as a step toward development of AIDS vaccines.
- Center for Molecular Immunology, Institute of Microbiology, Chinese Academy of Sciences, 13 Beiyitiao, Zhongguancun, Beijing 100080, People's Republic of China.
Organizational Affiliation: