DNA polymerases: Hoogsteen base-pairing in DNA replication?
Wang, J.(2005) Nature 437: E6-7; discussion E7
- PubMed: 16163299
- DOI: https://doi.org/10.1038/nature04199
- Primary Citation of Related Structures:
1ZET - PubMed Abstract:
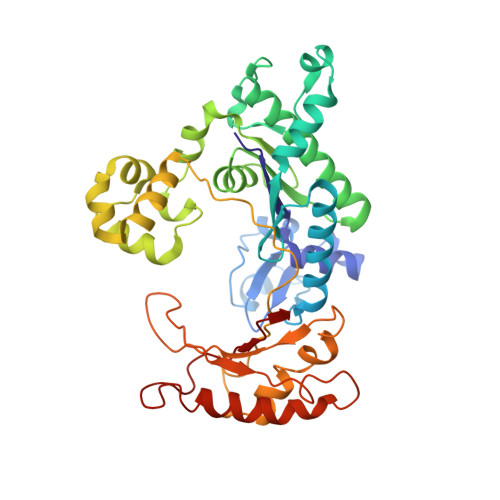
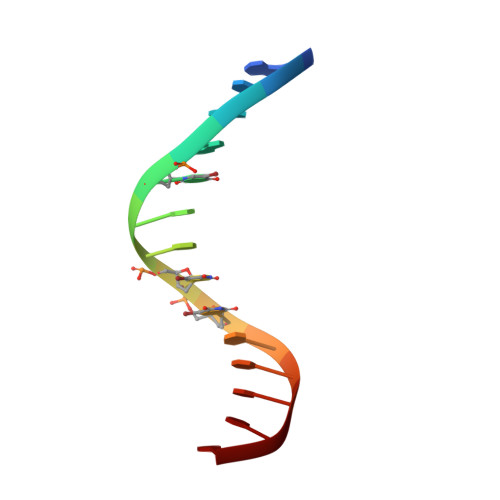
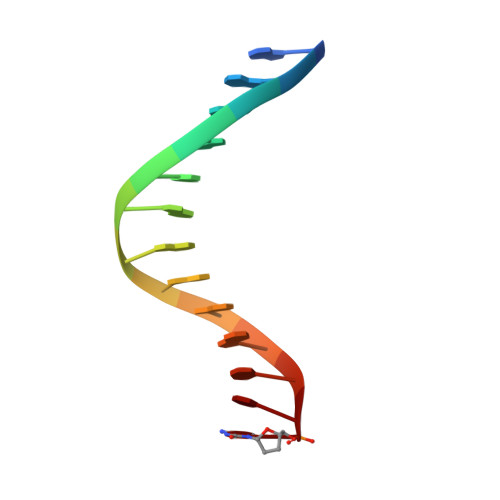
Human polymerase-iota belongs to the error-prone Y family of polymerases, which frequently incorporate incorrect nucleotides during DNA replication but can efficiently bypass DNA lesions. On the basis of X-ray diffraction data, Nair et al. propose that Hoogsteen base-pairing is adopted by DNA during its replication by this enzyme. Here I re-examine their X-ray data and find that the electron density is very weak for a Hoogsteen base pair formed between a template adenine deoxyribonucleotide in the syn conformation and a deoxythymidine 5'-triphosphate (dTTP), and that the fit is better for a normal Watson-Crick base pair. As a guanine-cytosine (G-C) base pair has no potential to form a Hoogsteen base pair at physiological pH, Hoogsteen base-pairing is unlikely to be used in replication by this polymerase.
- Center for Structural Biology, Department of Molecular Biophysics and Biochemistry, Yale University, New Haven, Connecticut 06520, USA wang@csb.yale.edu.
Organizational Affiliation: