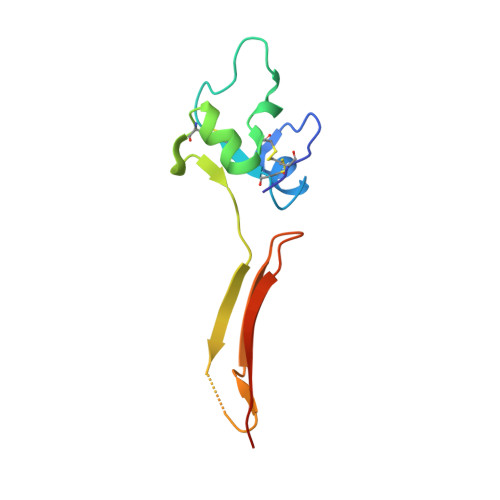
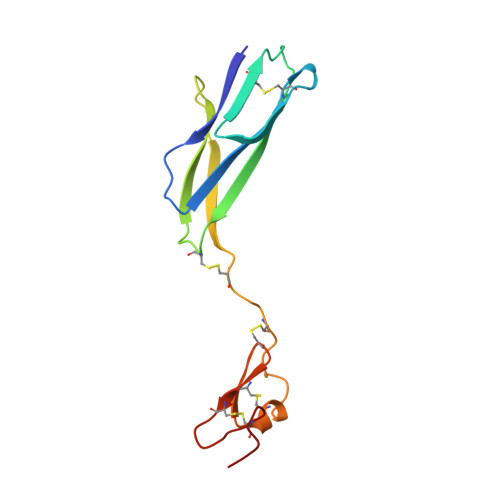
The Crystal Structure of the Plexin-Semaphorin-Integrin Domain/Hybrid Domain/I-EGF1 Segment from the Human Integrin {beta}2 Subunit at 1.8-A Resolution
Shi, M., Sundramurthy, K., Liu, B., Tan, S.M., Law, S.K., Lescar, J.(2005) J Biological Chem 280: 30586-30593
- PubMed: 15965234
- DOI: https://doi.org/10.1074/jbc.M502525200
- Primary Citation of Related Structures:
1YUK - PubMed Abstract:
Integrins are modular (alphabeta) heterodimeric proteins that mediate cell adhesion and convey signals across the plasma membrane. Interdomain motions play a key role in signal transduction by propagating structural changes through the molecule, thus controlling the activation state and adhesive properties of the integrin. We expressed a soluble fragment of the human integrin beta2 subunit comprising the plexin-semaphorin-integrin domain (PSI)/hybrid domain/I-EGF1 fragment and present its crystal structure at 1.8-A resolution. The structure reveals an elongated molecule with a rigid architecture stabilized by nine disulfide bridges. The PSI domain is located centrally and participates in the formation of extended interfaces with the hybrid domain and I-EGF1 domains, respectively. The hybrid domain/PSI interface involves the burial of an Arg residue, and contacts between PSI and I-EGF1 are mainly mediated by well conserved Arg and Trp residues. Conservation of key interacting residues across the various integrin beta subunits sequences suggests that our structure represents a good model for the entire integrin family. Superposition with the integrin beta3 receptor in its bent conformation suggests that an articulation point is present at the linkage between its I-EGF1 and I-EGF2 modules and underlines the importance of this region for the control of integrin-mediated cell adhesion.
- School of Biological Sciences, Nanyang Technological University, 60 Nanyang Drive, Singapore 637551, Singapore.
Organizational Affiliation: