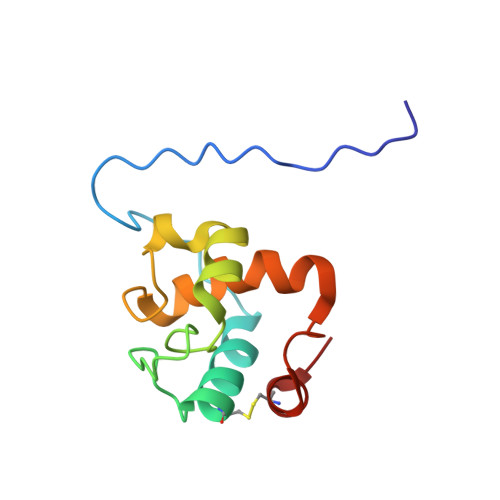
The structure of a resuscitation-promoting factor domain from Mycobacterium tuberculosis shows homology to lysozymes
Cohen-Gonsaud, M., Barthe, P., Bagneris, C., Henderson, B., Ward, J., Roumestand, C., Keep, N.H.(2005) Nat Struct Mol Biol 12: 270-273
- PubMed: 15723078
- DOI: https://doi.org/10.1038/nsmb905
- Primary Citation of Related Structures:
1XSF - PubMed Abstract:
Resuscitation-promoting factor (RPF) proteins reactivate stationary-phase cultures of (G+C)-rich Gram-positive bacteria including the causative agent of tuberculosis, Mycobacterium tuberculosis. We report the solution structure of the RPF domain from M. tuberculosis Rv1009 (RpfB) solved by heteronuclear multidimensional NMR. Structural homology with various glycoside hydrolases suggested that RpfB cleaved oligosaccharides. Biochemical studies indicate that a conserved active site glutamate is important for resuscitation activity. These data, as well as the presence of a clear binding pocket for a large molecule, indicate that oligosaccharide cleavage is probably the signal for revival from dormancy.
- School of Crystallography and Institute for Structural Molecular Biology, Birkbeck College, University of London, Malet Street, London, WC1E 7HX, UK.
Organizational Affiliation: