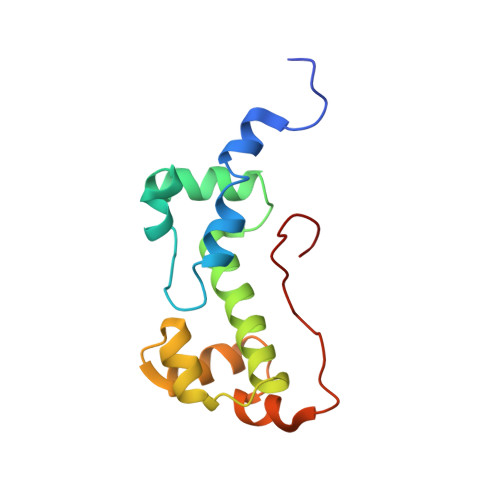
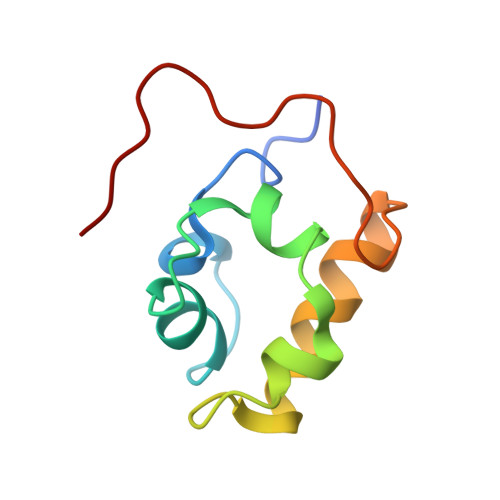
Versatility of the carboxy-terminal domain of the alpha subunit of RNA polymerase in transcriptional activation: use of the DNA contact site as a protein contact site for MarA.
Dangi, B., Gronenborn, A.M., Rosner, J.L., Martin, R.G.(2004) Mol Microbiol 54: 45-59
- PubMed: 15458404
- DOI: https://doi.org/10.1111/j.1365-2958.2004.04250.x
- Primary Citation of Related Structures:
1XS9 - PubMed Abstract:
The transcriptional activator, MarA, interacts with RNA polymerase (RNAP) to activate promoters of the mar regulon. Here, we identify the interacting surfaces of MarA and of the carboxy-terminal domain of the alpha subunit of RNAP (alpha-CTD) by NMR-based chemical shift mapping. Spectral changes were monitored for a MarA-DNA complex upon titration with alpha-CTD, and for alpha-CTD upon titration with MarA-DNA. The mapping results were confirmed by mutational studies and retention chromatography. A model of the ternary complex shows that alpha-CTD uses a '265-like determinant' to contact MarA at a surface distant from the DNA. This is unlike the interaction of alpha-CTD with the CRP or Fis activators where the '265 determinant' contacts DNA while another surface of the same alpha-CTD molecule contacts the activator. These results reveal a new versatility for alpha-CTD in transcriptional activation.
- Laboratory of Chemical Physics, National Institute of Diabetes, Digestive and Kidney Diseases, National Institute of Health, Bethesda, MD 20892, USA.
Organizational Affiliation: