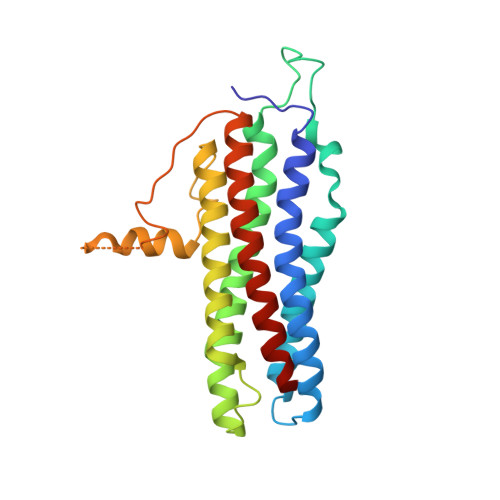
She2p is a novel RNA binding protein with a basic helical hairpin motif
Niessing, D., Huettelmaier, S., Zenklusen, D., Singer, R.H., Burley, S.K.(2004) Cell 119: 491-502
- PubMed: 15537539
- DOI: https://doi.org/10.1016/j.cell.2004.10.018
- Primary Citation of Related Structures:
1XLY - PubMed Abstract:
Selective transport of mRNAs in ribonucleoprotein particles (mRNP) ensures asymmetric distribution of information within and among eukaryotic cells. Actin-dependent transport of ASH1 mRNA in yeast represents one of the best-characterized examples of mRNP translocation. Formation of the ASH1 mRNP requires recognition of zip code elements by the RNA binding protein She2p. We determined the X-ray structure of She2p at 1.95 A resolution. She2p is a member of a previously unknown class of nucleic acid binding proteins, composed of a single globular domain with a five alpha helix bundle that forms a symmetric homodimer. After demonstrating potent, dimer-dependent RNA binding in vitro, we mapped the RNA binding surface of She2p to a basic helical hairpin in vitro and in vivo and present a mechanism for mRNA-dependent initiation of ASH1 mRNP complex assembly.
- Laboratories of Molecular Biophysics and The Rockefeller University, 1230 York Avenue, New York, NY 10021, USA.
Organizational Affiliation: