Structure of plasmepsin II in complex of an pepstatin analogue
Prade, L.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Plasmepsin 2 | 329 | Plasmodium falciparum | Mutation(s): 0 EC: 3.4.23.39 | 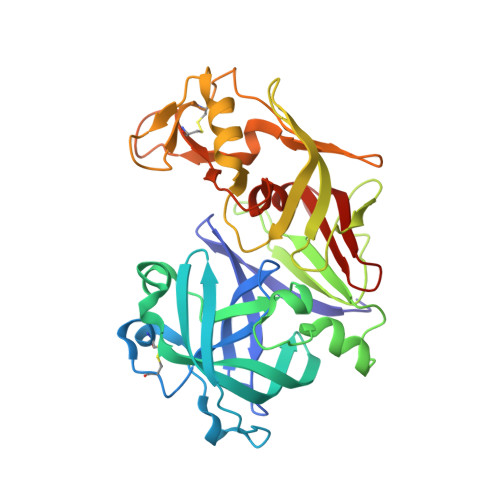 | |
UniProt | |||||
Find proteins for P46925 (Plasmodium falciparum (isolate HB3)) Explore P46925 Go to UniProtKB: P46925 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P46925 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| 5FP Query on 5FP | C [auth A], D [auth B] | 5,5,5-TRIFLUORO-3-HYDROXY-4-[2-(5,5,5-TRIFLUORO-3-HYDROXY-4-{3-METHYL-2-[3-METHYL-2-(3-METHYL-BUTYRYLAMINO)-BUTYRYLAMINO]-BUTYRYLAMINO}-PENTANOYLAMINO)-PROPIONYLAMINO]-PENTANOIC ACID C28 H45 F6 N5 O9 UDWBPCUKJLZZFA-AIBCRYBESA-N |  | ||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| ID | Chains | Name | Type/Class | 2D Diagram | 3D Interactions |
| PRD_000640 (5FP) Query on PRD_000640 | C [auth A], D [auth B] | bis-trifluoromethyl pepstatin A acid analogue | Peptide-like / Inhibitor |  | |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 141.154 | α = 90 |
| b = 141.154 | β = 90 |
| c = 97.375 | γ = 120 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| MOSFLM | data reduction |
| CCP4 | data scaling |
| MOLREP | phasing |