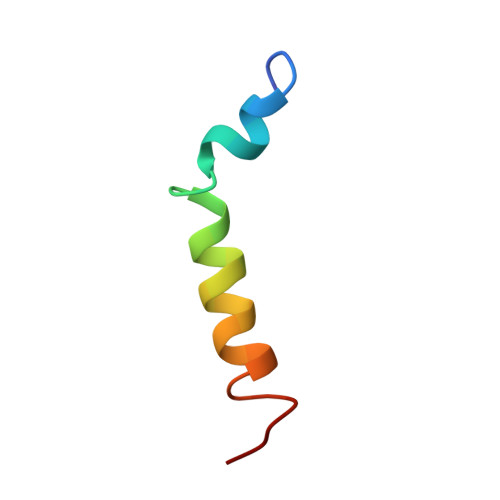
Structure and orientation of pardaxin determined by NMR experiments in model membranes
Porcelli, F., Buck, B., Lee, D.-K., Hallock, K.J., Ramamoorthy, A., Veglia, G.(2004) J Biological Chem 279: 45815-45823
- PubMed: 15292173
- DOI: https://doi.org/10.1074/jbc.M405454200
- Primary Citation of Related Structures:
1XC0 - PubMed Abstract:
Pardaxins are a class of ichthyotoxic peptides isolated from fish mucous glands. Pardaxins physically interact with cell membranes by forming pores or voltage-gated ion channels that disrupt cellular functions. Here we report the high-resolution structure of synthetic pardaxin Pa4 in sodium dodecylphosphocholine micelles, as determined by (1)H solution NMR spectroscopy. The peptide adopts a bend-helix-bend-helix motif with an angle between the two structure helices of 122 +/- 9 degrees , making this structure substantially different from the one previously determined in organic solvents. In addition, paramagnetic solution NMR experiments on Pa4 in micelles reveal that except for the C terminus, the peptide is not solvent-exposed. These results are complemented by solid-state NMR experiments on Pa4 in lipid bilayers. In particular, (13)C-(15)N rotational echo double-resonance experiments in multilamellar vesicles support the helical conformation of the C-terminal segment, whereas (2)H NMR experiments show that the peptide induces considerable disorder in both the head-groups and the hydrophobic core of the bilayers. These solid-state NMR studies indicate that the C-terminal helix has a transmembrane orientation in DMPC bilayers, whereas in POPC bilayers, this domain is heterogeneously oriented on the lipid surface and undergoes slow motion on the NMR time scale. These new data help explain how the non-covalent interactions of Pa4 with lipid membranes induce a stable secondary structure and provide an atomic view of the membrane insertion process of Pa4.
- Department of Chemistry, University of Minnesota, Minneapolis, Minnesota 55455, USA.
Organizational Affiliation: