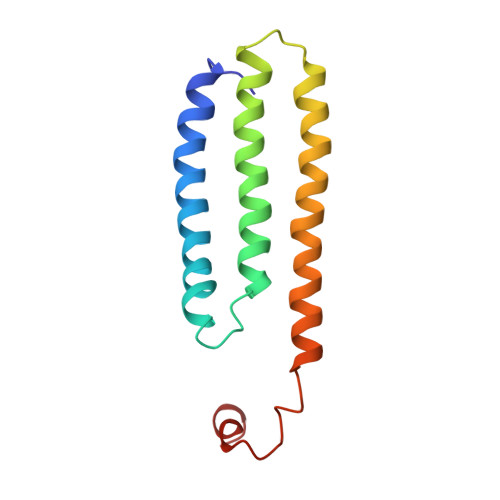
Solution Structure of Choline Binding Protein A, the Major Adhesin of Streptococcus Pneumoniae
Luo, R., Mann, B., Lewis, W.S., Rowe, A., Heath, R., Stewart, M.L., Hamburger, A.E., Sivakolundu, S., Lacy, E.R., Bjorkman, P.J., Tuomanen, E., Kriwacki, R.W.(2005) EMBO J 24: 34
- PubMed: 15616594
- DOI: https://doi.org/10.1038/sj.emboj.7600490
- Primary Citation of Related Structures:
1W9R - PubMed Abstract:
Streptococcus pneumoniae (pneumococcus) remains a significant health threat worldwide, especially to the young and old. While some of the biomolecules involved in pneumococcal pathogenesis are known and understood in mechanistic terms, little is known about the molecular details of bacterium/host interactions. We report here the solution structure of the 'repeated' adhesion domains (domains R1 and R2) of the principal pneumococcal adhesin, choline binding protein A (CbpA). Further, we provide insights into the mechanism by which CbpA binds its human receptor, polymeric immunoglobulin receptor (pIgR). The R domains, comprised of 12 imperfect copies of the leucine zipper heptad motif, adopt a unique 3-alpha-helix, raft-like structure. Each pair of alpha-helices is antiparallel and conserved residues in the loop between Helices 1 and 2 exhibit a novel 'tyrosine fork' structure that is involved in binding pIgR. This and other structural features that we show are conserved in most pneumococcal strains appear to generally play an important role in bacterial adhesion to pIgR. Interestingly, pneumococcus is the only bacterium known to adhere to and invade human cells by binding to pIgR.
- Department of Structural Biology, St Jude Children's Research Hospital, Memphis, TN 38105, USA.
Organizational Affiliation: