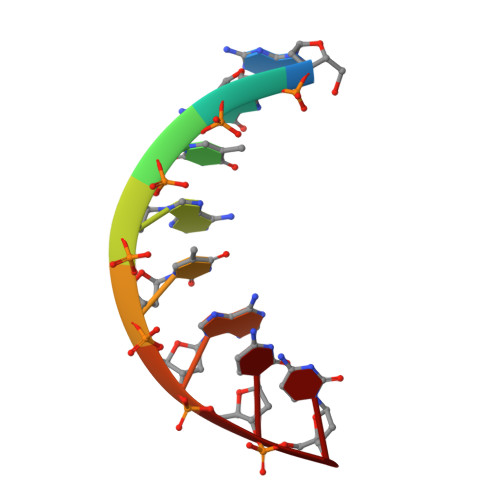
Sequence-dependent conformation of an A-DNA double helix: The crystal structure of the octamer d(G-G-T-A-T-A-C-C)
Shakked, Z., Rabinovich, D., Kennard, O., Cruse, W.B., Salisbury, S.A., Viswamitra, M.A.(1983) J Mol Biology 166: 183-201
- PubMed: 6854642
- DOI: https://doi.org/10.1016/s0022-2836(83)80005-9
- Primary Citation of Related Structures:
1VJ4 - PubMed Abstract:
The crystal structures of the synthetic self-complementary octamer d(G-G-T-A-T-A-C-C) and its 5-bromouracil-containing analogue have been refined to R values of 20% and 14% at resolutions of 1.8 and 2.25 A, respectively. The molecules adopt and A-DNA type double-helical conformation, which is minimally affected by crystal forces. A detailed analysis of the structure shows a considerable influence of the nucleotide sequence on the base-pair stacking patterns. In particular, the electrostatic stacking interactions between adjacent guanine and thymine bases produce symmetric bending of the double helix and a major-groove widening. The sugar-phosphate backbone appears to be only slightly affected by the base sequence. The local variations in the base-pair orientation are brought about by correlated adjustments in the backbone torsion angles and the glycosidic orientation. Sequence-dependent conformational variations of the type observed here may contribute to the specificity of certain protein-DNA interactions.