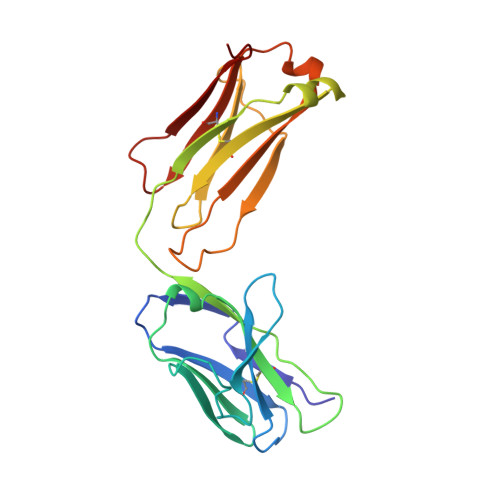
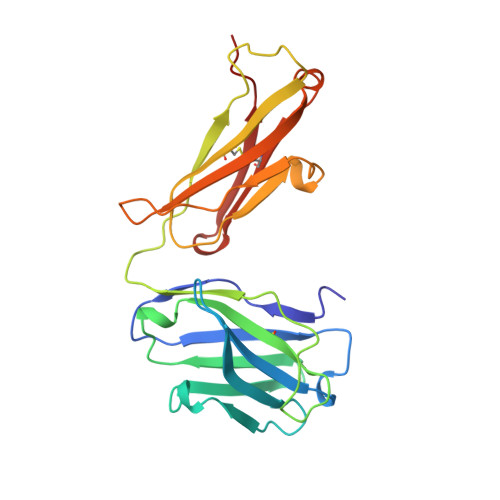
Structural studies of human autoantibodies. Crystal structure of a thyroid peroxidase autoantibody Fab.
Chacko, S., Padlan, E.A., Portolano, S., McLachlan, S.M., Rapoport, B.(1996) J Biological Chem 271: 12191-12198
- PubMed: 8647813
- DOI: https://doi.org/10.1074/jbc.271.21.12191
- Primary Citation of Related Structures:
1VGE - PubMed Abstract:
The three-dimensional structure of the Fab of TR1.9, a high-affinity IgG1, kappa human autoantibody to thyroid peroxidase, was determined crystallographically to a resolution of 2.0 A. The combining site was found to be relatively flat, like other antibodies to large proteins. Sequence differences from the most closely related germline genes mainly occur at positions occupied by residues with outward-pointing side chains. An increased deformability of the second and third complementarity-determining regions of the heavy chain may result from the replacement of two germline asparagines and the presence of several glycines, and may allow "induced fit" in the binding to antigen. Four exposed charged residues, resulting from the use of a particular D (diversity) and J (joining) segments in the assembly of the heavy chain, may contribute to the high affinity of antigen binding. The crystal structure of TR1.9 Fab is the first for a human IgG high-affinity autoantibody.
- Laboratory of Molecular Biology, NIDDK, National Institutes of Health, Bethesda, Maryland 20895-0560, USA.
Organizational Affiliation: