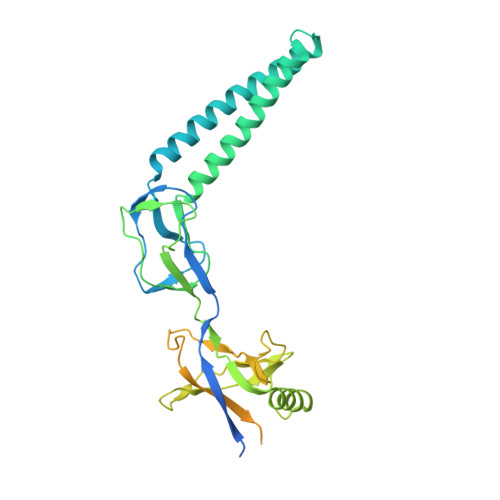
Crystal structure of the membrane fusion protein, MexA, of the multidrug transporter in Pseudomonas aeruginosa
Akama, H., Matsuura, T., Kashiwagi, S., Yoneyama, H., Narita, S., Tsukihara, T., Nakagawa, A., Nakae, T.(2004) J Biological Chem 279: 25939-25942
- PubMed: 15117957
- DOI: https://doi.org/10.1074/jbc.C400164200
- Primary Citation of Related Structures:
1VF7 - PubMed Abstract:
The MexAB-OprM efflux pump of Pseudomonas aeruginosa is central to multidrug resistance of this organism, which infects immunocompromised hospital patients. The MexA, MexB, and OprM subunits were assumed to function as the membrane fusion protein, the body of the transporter, and the outer membrane channel protein, respectively. For better understanding of this important xenobiotic transporter, we show the x-ray crystallographic structure of MexA at a resolution of 2.40 A. The global MexA structure showed unforeseen new features with a spiral assembly of six and seven protomers that were joined together at one end by a pseudo 2-fold image. The protomer showed a new protein structure with a tandem arrangement consisting of at least three domains and presumably one more. The rod domain had a long hairpin of twisted coiled-coil that extended to one end. The second domain adjacent to the rod alpha-helical domain was globular and constructed by a cluster of eight short beta-sheets. The third domain located distal to the alpha-helical rod was globular and composed of seven short beta-sheets and one short alpha-helix. The 13-mer was shaped like a woven rattan cylinder with a large internal tubular space and widely opened flared ends. The 6-mer and 7-mer had a funnel-like structure consisting of a tubular rod at one side and a widely opened flared funnel top at the other side. Based on these results, we constructed a model of the MexAB-OprM pump assembly. The three pairs of MexA dimers interacted with the periplasmic alpha-barrel domain of OprM via the alpha-helical hairpin, the second domain interacted with both MexB and OprM at their contact site, and the third and disordered domains probably interacted with the distal domain of MexB. In this fashion, the MexA subunit connected MexB and OprM, indicating that MexA is the membrane bridge protein.
- Department of Molecular Life Science, Tokai University School of Medicine, Isehara, 259-1193, Japan.
Organizational Affiliation: