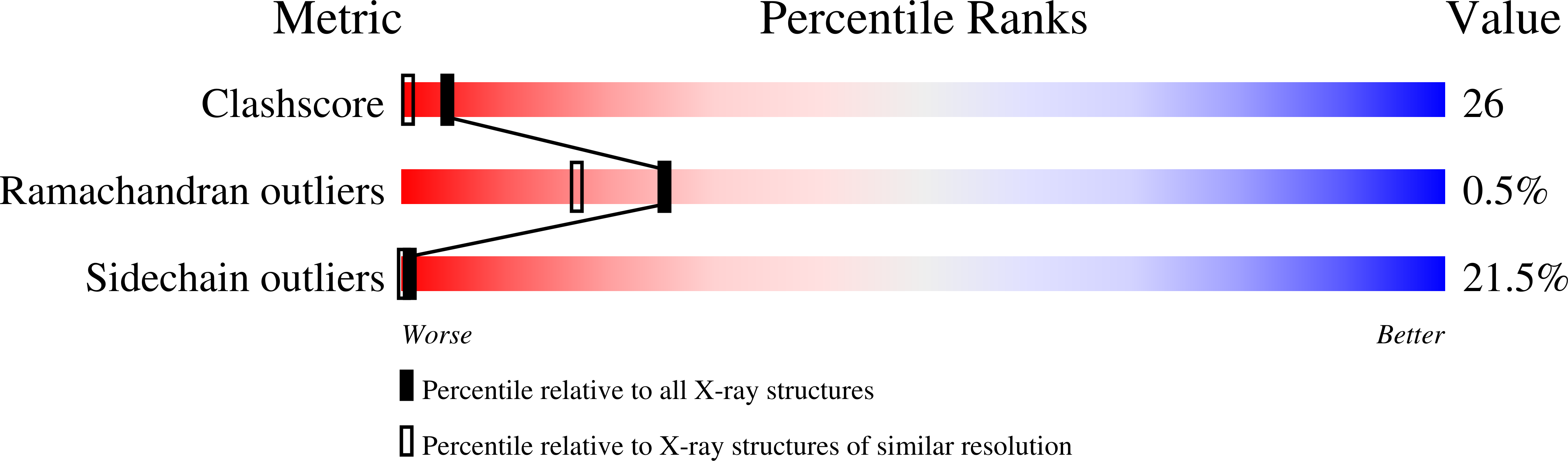The crystal structure of a five-stranded coiled coil in COMP: a prototype ion channel?
Malashkevich, V.N., Kammerer, R.A., Efimov, V.P., Schulthess, T., Engel, J.(1996) Science 274: 761-765
- PubMed: 8864111
- DOI: https://doi.org/10.1126/science.274.5288.761
- Primary Citation of Related Structures:
1VDF - PubMed Abstract:
Oligomerization by the formation of alpha-helical bundles is common in many proteins. The crystal structure of a parallel pentameric coiled coil, constituting the oligomerization domain in the cartilage oligomeric matrix protein (COMP), was determined at 2.05 angstroms resolution. The same structure probably occurs in two other extracellular matrix proteins, thrombospondins 3 and 4. Complementary hydrophobic interactions and conserved disulfide bridges between the alpha helices result in a thermostable structure with unusual properties. The long hydrophobic axial pore is filled with water molecules but can also accommodate small apolar groups. An "ion trap" is formed inside the pore by a ring of conserved glutamines, which binds chloride and probably other monatomic anions. The oligomerization domain of COMP has marked similarities with proposed models of the pentameric transmembrane ion channels in phospholamban and the acetylcholine receptor.
- Department of Structural Biology, Biozentrum, University of Basel, Klingelbergstrasse 70, CH-4056 Basel, Switzerland.
Organizational Affiliation: