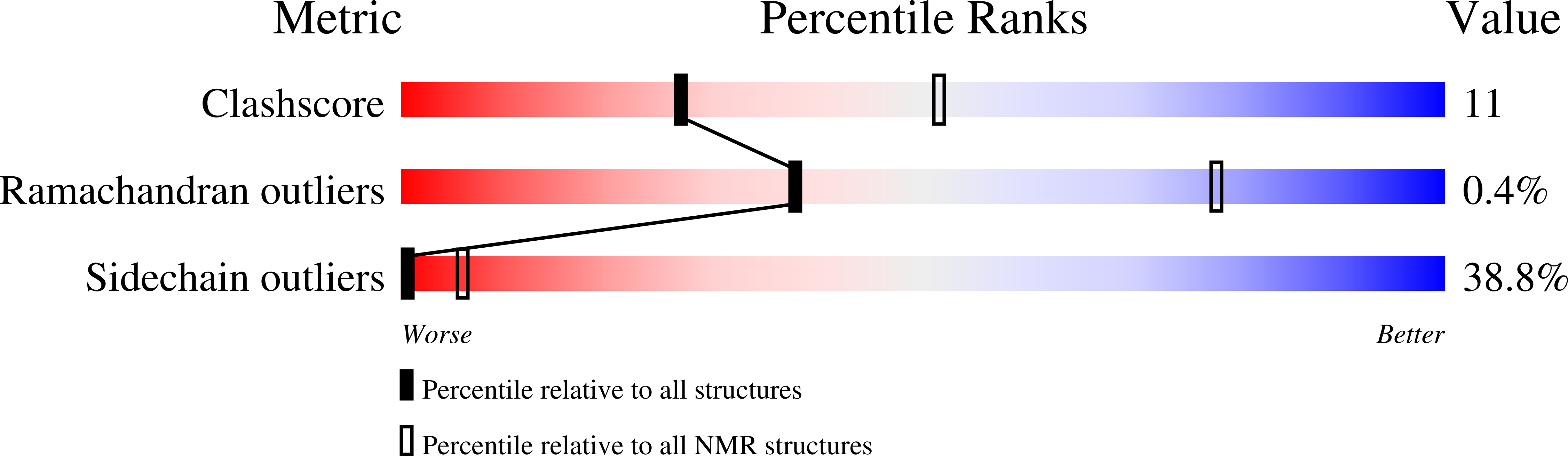Tissue-specific expression of head-to-tail cyclized miniproteins in Violaceae and structure determination of the root cyclotide Viola hederacea root cyclotide1
Trabi, M., Craik, D.J.(2004) Plant Cell 16: 2204-2216
- PubMed: 15295104
- DOI: https://doi.org/10.1105/tpc.104.021790
- Primary Citation of Related Structures:
1VB8 - PubMed Abstract:
The plant cyclotides are a family of 28 to 37 amino acid miniproteins characterized by their head-to-tail cyclized peptide backbone and six absolutely conserved Cys residues arranged in a cystine knot motif: two disulfide bonds and the connecting backbone segments form a loop that is penetrated by the third disulfide bond. This knotted disulfide arrangement, together with the cyclic peptide backbone, renders the cyclotides extremely stable against enzymatic digest as well as thermal degradation, making them interesting targets for both pharmaceutical and agrochemical applications. We have examined the expression patterns of these fascinating peptides in various Viola species (Violaceae). All tissue types examined contained complex mixtures of cyclotides, with individual profiles differing significantly. We provide evidence for at least 57 novel cyclotides present in a single Viola species (Viola hederacea). Furthermore, we have isolated one cyclotide expressed only in underground parts of V. hederacea and characterized its primary and three-dimensional structure. We propose that cyclotides constitute a new family of plant defense peptides, which might constitute an even larger and, in their biological function, more diverse family than the well-known plant defensins.
- Institute for Molecular Bioscience, University of Queensland, Brisbane, Queensland 4072, Australia.
Organizational Affiliation: