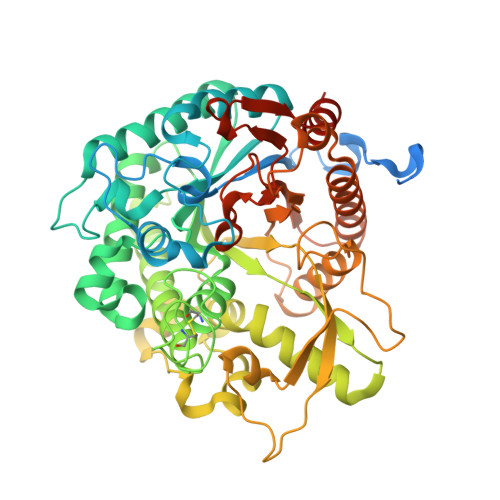
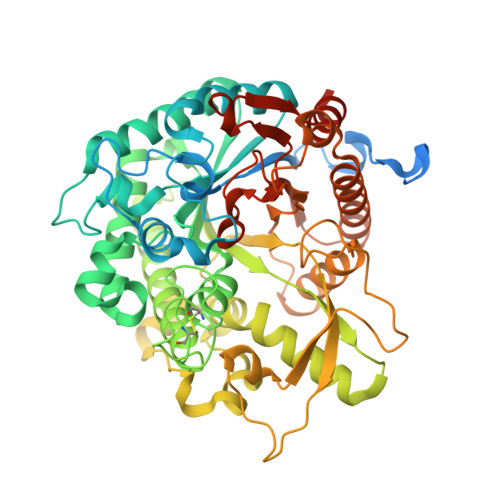
Structural Determinants of Substrate Specificity in Family 1 Beta-Glucosidases: Novel Insights from the Crystal Structure of Sorghum Dhurrinase-1, a Plant Beta-Glucosidase with Strict Specificity, in Complex with its Natural Substrate
Verdoucq, L., Moriniere, J., Bevan, D.R., Esen, A., Vasella, A., Henrissat, B., Czjzek, M.(2004) J Biological Chem 279: 31796
- PubMed: 15148317
- DOI: https://doi.org/10.1074/jbc.M402918200
- Primary Citation of Related Structures:
1V02, 1V03, 1V08 - PubMed Abstract:
Plant beta-glucosidases play a crucial role in defense against pests. They cleave, with variable specificity, beta-glucosides to release toxic aglycone moieties. The Sorghum bicolor beta-glucosidase isoenzyme Dhr1 has a strict specificity for its natural substrate dhurrin (p-hydroxy-(S)-mandelonitrile-beta-D-glucoside), whereas its close homolog, the maize beta-glucosidase isoenzyme Glu1, which shares 72% sequence identity, hydrolyzes a broad spectrum of substrates in addition to its natural substrate 2-O-beta-D-glucopyranosyl-4-hydroxy-7-methoxy-1,4-benzoxaxin-3-one. Structural data from enzyme.substrate complexes of Dhr1 show that the mode of aglycone binding differs from that previously observed in the homologous maize enzyme. Specifically, the data suggest that Asn(259), Phe(261), and Ser(462), located in the aglycone-binding site of S. bicolor Dhr1, are crucial for aglycone recognition and binding. The tight binding of the aglycone moiety of dhurrin promotes the stabilization of the reaction intermediate in which the glycone moiety is in a deformed (1)S(3) conformation within the glycone-binding site, ready for nucleophilic attack to occur. Compared with the broad specificity maize beta-glucosidase, this different binding mode explains the narrow specificity of sorghum dhurrinase-1.
- Department of Biology, Virginia Polytechnic Institute and State University, Blacksburg, Virginia 24061, USA.
Organizational Affiliation: