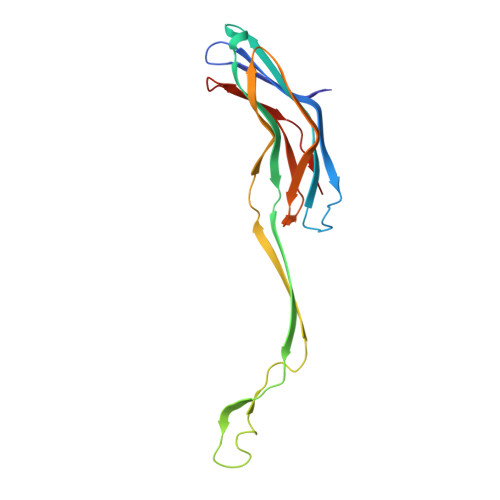
The Structure of a Mycobacterial Outer-Membrane Channel
Faller, M., Niederweis, M., Schulz, G.E.(2004) Science 303: 1189
- PubMed: 14976314
- DOI: https://doi.org/10.1126/science.1094114
- Primary Citation of Related Structures:
1UUN - PubMed Abstract:
Mycobacteria have low-permeability outer membranes that render them resistant to most antibiotics. Hydrophilic nutrients can enter by way of transmembrane-channel proteins called porins. An x-ray analysis of the main porin from Mycobacterium smegmatis, MspA, revealed a homooctameric goblet-like conformation with a single central channel. This is the first structure of a mycobacterial outer-membrane protein. No structure-related protein was found in the Protein Data Bank. MspA contains two consecutive beta barrels with nonpolar outer surfaces that form a ribbon around the porin, which is too narrow to fit the thickness of the mycobacterial outer membrane in contemporary models.
- Institut für Organische Chemie und Biochemie, Albert-Ludwigs-Universität, Albertstrasse 21, 79104 Freiburg im Breisgau, Germany.
Organizational Affiliation: