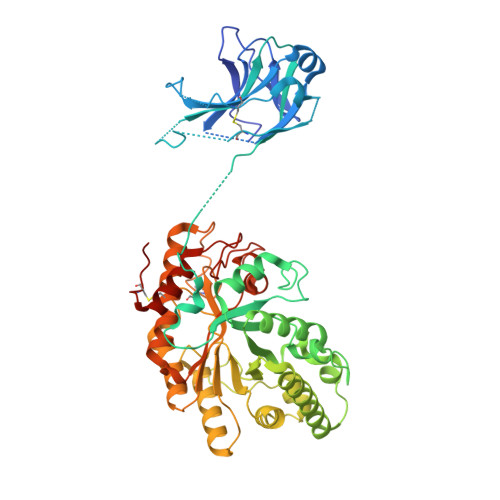
Structural and Biochemical Analysis of Cellvibrio Japonicus Xylanase 10C: How Variation in Substrate-Binding Cleft Influences the Catalytic Profile of Family Gh-10 Xylanases
Pell, G., Szabo, L., Charnock, S.J., Xie, H., Gloster, T.M., Davies, G.J., Gilbert, H.J.(2004) J Biological Chem 279: 11777
- PubMed: 14670951
- DOI: https://doi.org/10.1074/jbc.M311947200
- Primary Citation of Related Structures:
1US2, 1US3 - PubMed Abstract:
Microbial degradation of the plant cell wall is the primary mechanism by which carbon is utilized in the biosphere. The hydrolysis of xylan, by endo-beta-1,4-xylanases (xylanases), is one of the key reactions in this process. Although amino acid sequence variations are evident in the substrate binding cleft of "family GH10" xylanases (see afmb.cnrs-mrs.fr/CAZY/), their biochemical significance is unclear. The Cellvibrio japonicus GH10 xylanase CjXyn10C is a bi-modular enzyme comprising a GH10 catalytic module and a family 15 carbohydrate-binding module. The three-dimensional structure at 1.85 A, presented here, shows that the sequence joining the two modules is disordered, confirming that linker sequences in modular glycoside hydrolases are highly flexible. CjXyn10C hydrolyzes xylan at a rate similar to other previously described GH10 enzymes but displays very low activity against xylooligosaccharides. The poor activity on short substrates reflects weak binding at the -2 subsite of the enzyme. Comparison of CjXyn10C with other family GH10 enzymes reveals "polymorphisms" in the substrate binding cleft including a glutamate/glycine substitution at the -2 subsite and a tyrosine insertion in the -2/-3 glycone region of the substrate binding cleft, both of which contribute to the unusual properties of the enzyme. The CjXyn10C-substrate complex shows that Tyr-340 stacks against the xylose residue located at the -3 subsite, and the properties of Y340A support the view that this tyrosine plays a pivotal role in substrate binding at this location. The generic importance of using CjXyn10C as a template in predicting the biochemical properties of GH10 xylanases is discussed.
- School of Cell and Molecular Biosciences, University of Newcastle upon Tyne, The Agriculture Bldg., Newcastle upon Tyne NE1 7RU.
Organizational Affiliation: