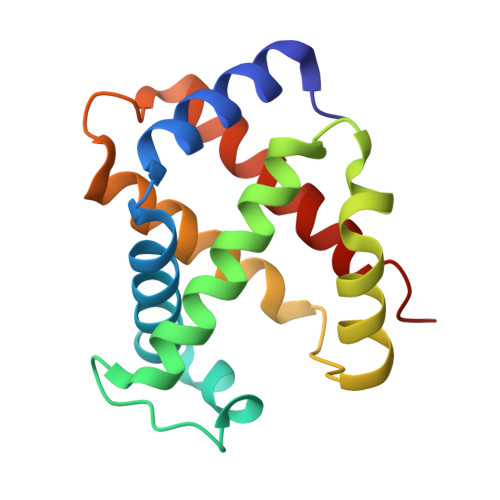
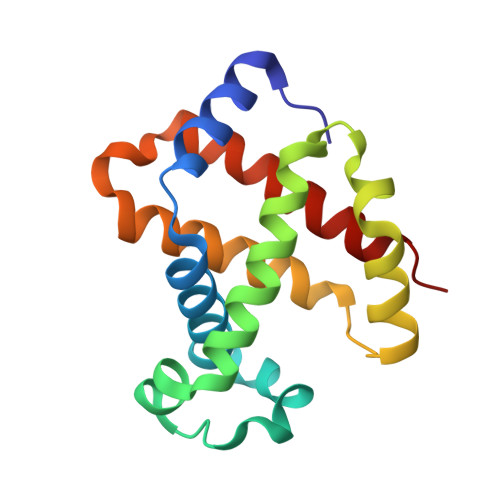
Crystal structures of unliganded and half-liganded human hemoglobin derivatives cross-linked between Lys 82beta1 and Lys 82beta2
Park, S.-Y., Shibayama, N., Hiraki, T., Tame, J.R.H.(2004) Biochemistry 43: 8711-8717
- PubMed: 15236579
- DOI: https://doi.org/10.1021/bi049932w
- Primary Citation of Related Structures:
1UIW - PubMed Abstract:
A number of ligand binding studies of human adult hemoglobin (HbA) cross-linked between Lys 82beta(1) and Lys 82beta(2) with bis(3,5-dibromosalicyl)fumarate have been reported. The oxygen binding properties of native HbA, including the cooperativity and Bohr effect, are not substantially changed by the modification, provided care is taken to remove electrophoretically silent impurities arising from side reactions. We have refined the high-resolution structure of this modified Hb and found it adopts the T state when crystallized in the absence of heme ligands, contrary to a previously published structure. These results suggest the slightly altered crystal form determined previously may be due to unremoved side products of the cross-linking reaction with high oxygen affinity. Two nickel-substituted Hbs cross-linked in the same way have also been crystallized in the presence of carbon monoxide, which binds only to the ferrous heme. In the case of the nickel-substituted alpha subunit, the absence of a covalent link between the central metal of the heme and the proximal histidine leads to a new conformation of the histidine stabilized by a water molecule. This structure may mimic that of partially NO-liganded species of HbA; however, overall, the changes are highly localized, and both doubly ligated species are in the T conformation.
- Protein Design Laboratory, Graduate School of Integrated Science, Yokohama City University, 1-7-29 Suehiro, Tsurumi, Yokohama 230-0045, Japan.
Organizational Affiliation: