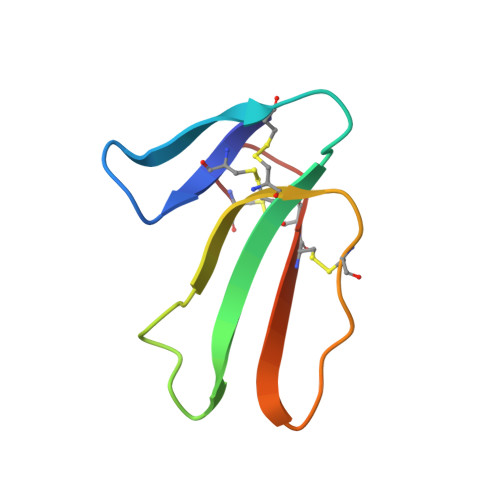
X-ray structure at 1.55 A of toxin gamma, a cardiotoxin from Naja nigricollis venom. Crystal packing reveals a model for insertion into membranes.
Bilwes, A., Rees, B., Moras, D., Menez, R., Menez, A.(1994) J Mol Biology 239: 122-136
- PubMed: 8196041
- DOI: https://doi.org/10.1006/jmbi.1994.1357
- Primary Citation of Related Structures:
1TGX - PubMed Abstract:
The crystal structure of toxin gamma from Naja nigricollis has been solved and refined to 1.55 A resolution. The final R-factor, computed with all X-ray data available, is 17.9%. The three-dimensional structure is characterized by a core formed by two beta-sheets organized in three extended loops. It is similar to that of cardiotoxin V4II from Naja mossambica mossambica, with the exception of the hydrophobic loop I. The flexibility and variability of the loops contrast sharply with the rigidity of the molecular core and its high degree of structural conservation among the cardiotoxin family. The most flexible loop II adopts different conformations in the three monomers forming the crystal asymmetric unit. These monomers form a trimer around an approximate 3-fold axis, with conserved hydrophobic side-chains on the outside and hydrophilic residues in the central channel or involved in interactions with the other molecules. The trimer thus resembles a membrane protein with a central channel that could allow the passage of small ions. It is proposed as a model for the insertion of cardiotoxin into a membrane.
- UPR de Biologie Structurale, Institut de Biologie Moléculaire et Cellulaire du CNRS, Strasbourg, France.
Organizational Affiliation: