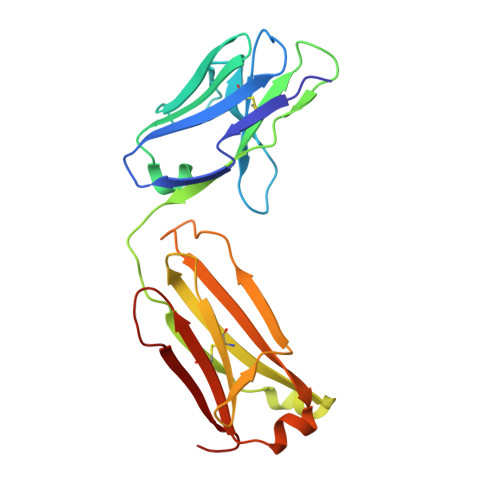
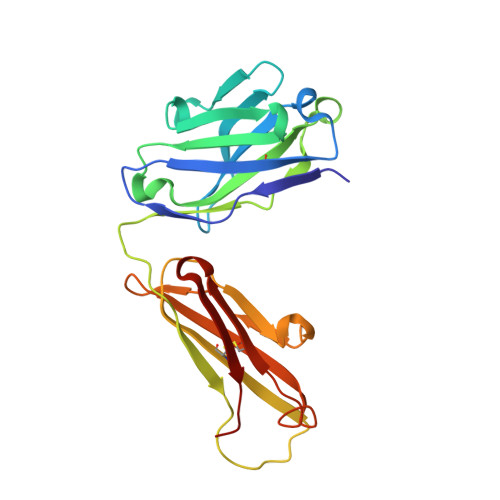
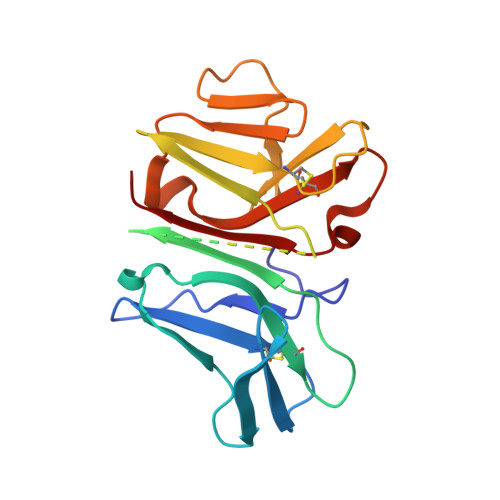
Crystal structure of the human T cell receptor CD3(epsilon)(gamma) heterodimer complexed to the therapeutic mAb OKT3.
Kjer-Nielsen, L., Dunstone, M.A., Kostenko, L., Ely, L.K., Beddoe, T., Mifsud, N.A., Purcell, A.W., Brooks, A.G., McCluskey, J., Rossjohn, J.(2004) Proc Natl Acad Sci U S A 101: 7675-7680
- PubMed: 15136729
- DOI: https://doi.org/10.1073/pnas.0402295101
- Primary Citation of Related Structures:
1SY6 - PubMed Abstract:
The CD3 epsilon gamma heterodimer is essential for expression and function of the T cell receptor. The crystal structure of the human CD3 epsilon gamma heterodimer is described to 2.1-A resolution complexed with OKT3, a therapeutic mAb that not only activates and tolerizes mature T cells but also induces regulatory T cells. The mode of CD3 epsilon gamma dimerization provides a general structural basis for CD3 assembly and maps candidate T cell antigen receptor docking sites, including a duplicated linear region rich in acidic residues that is unique to human CD3 epsilon. OKT3 binds to an atypically small area of CD3 epsilon and has a low affinity for the isolated CD3 epsilon gamma heterodimer. The structure of the OKT3/CD3 epsilon gamma complex has implications for T cell signaling and therapeutic design.
- Department of Microbiology and Immunology, University of Melbourne, Parkville, Victoria 3010, Australia.
Organizational Affiliation: