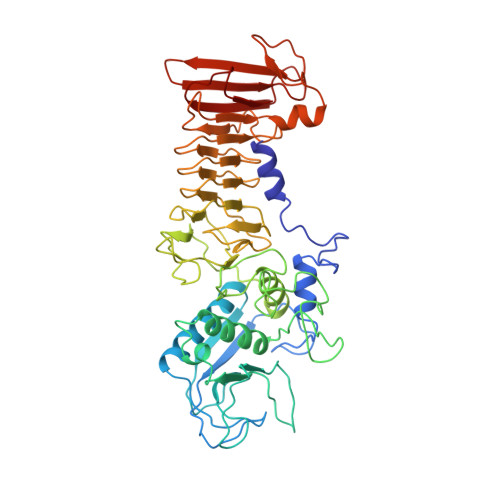
Crystal structure of Serratia protease, a zinc-dependent proteinase from Serratia sp. E-15, containing a beta-sheet coil motif at 2.0 A resolution.
Hamada, K., Hata, Y., Katsuya, Y., Hiramatsu, H., Fujiwara, T., Katsube, Y.(1996) J Biochem 119: 844-851
- PubMed: 8797082
- DOI: https://doi.org/10.1093/oxfordjournals.jbchem.a021320
- Primary Citation of Related Structures:
1SRP - PubMed Abstract:
The crystal structure of Serratia protease from Serratia sp. E-15 was solved by the single isomorphous replacement method supplemented with anomalous scattering effects from both the Zn atom in the native crystal and the Sm atom in the derivative crystal, and refined at 2.0 A resolution to a crystallographic R-factor of 0.194. The enzyme consists of N-terminal catalytic and C-terminal beta-sandwich domains, as observed in alkaline protease from Pseudomonas aeruginosa IFO3080. The catalytic domain with a five-stranded antiparallel beta-sheet and five alpha-helices shares a basically common folding topology with those of other zinc metalloendoproteases. The catalytic zinc ion at the bottom of the active site cleft is ligated by His176, His180, His186, Tyr216, and a water molecule in a distorted trigonalbipyramidal manner. The C-terminal domain is a beta-strand-rich domain containing eighteen beta-strands and a short alpha-helix, and has seven Ca2+ ions bound to calcium binding loops. An unusual beta-sheet coil motif is observed in this domain, where the beta-strands and calcium binding loops are alternately incorporated into an elliptical right-handed spiral so as to form a two-layer untwisted beta-sandwich structure. The Ca2+ ions in the C-terminal domain seem to be very important for the folding and stability of the beta-sheet coil structure.
- Faculty of Science, Shimane University. hamadak@cis.shimane.u.ac.jp
Organizational Affiliation: