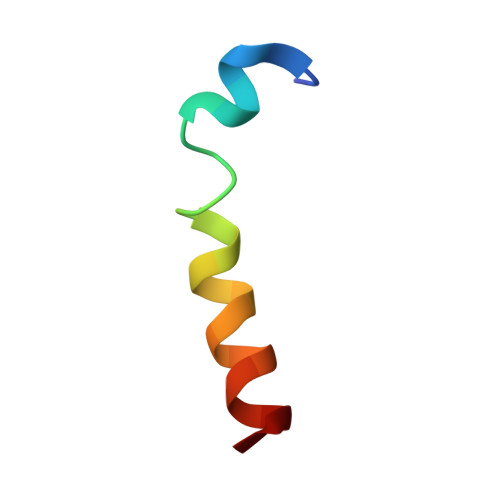
NMR solution structure and position of transportan in neutral phospholipid bicelles
Andersson, A.(2004) FEBS Lett 567: 265-269
- PubMed: 15178334
- DOI: https://doi.org/10.1016/j.febslet.2004.04.079
- Primary Citation of Related Structures:
1SMZ - PubMed Abstract:
Transportan is a chimeric cell-penetrating peptide constructed from the peptides galanin and mastoparan, which has the ability to internalize living cells carrying a hydrophilic load. In this study, we have determined the NMR solution structure and investigated the position of transportan in neutral bicelles. The structure revealed a well-defined alpha-helix in the C-terminal mastoparan part of the peptide and a weaker tendency to form an alpha-helix in the N-terminal domain. The position of the peptide in relation to the membrane, as studied by adding paramagnetic probes, shows that the peptide lies parallel to, and in the head-group region of the membrane surface. This result is supported by amide proton secondary chemical shifts.
- Department of Biochemistry and Biophysics, The Arrhenius Laboratories, Stockholm University, S-10691 Stockholm, Sweden.
Organizational Affiliation: