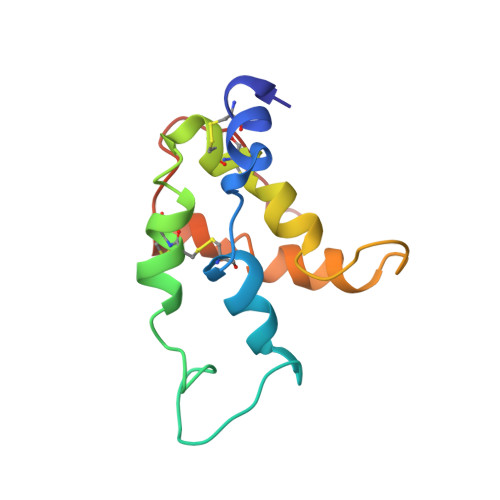
Solution structure and stability against digestion of rproBnIb, a recombinant 2S albumin from rapeseed: relationship to its allergenic properties.
Pantoja-Uceda, D., Palomares, O., Bruix, M., Villalba, M., Rodriguez, R., Rico, M., Santoro, J.(2004) Biochemistry 43: 16036-16045
- PubMed: 15609998
- DOI: https://doi.org/10.1021/bi048069x
- Primary Citation of Related Structures:
1SM7 - PubMed Abstract:
NMR spectroscopy has been used to determine the solution structure of the precursor form of the recombinant napin BnIb, rproBnIb, a 2S albumin, 109-residue protein from the seeds of Brassica napus. More than 90% of the side-chain proton resonances were unambiguously assigned from the analysis of two-dimensional correlation (COSY), total correlation (TOCSY), and nuclear Overhauser effect (NOESY) spectra. The final structures were computed by using restrained molecular dynamics on the basis of 1316 upper-limit distance constraints derived from NOE cross-correlation intensities. The computed structures exhibited a root-mean-square deviation (RMSD) radius of 0.66 A for the backbone and 1.16 A for the side-chain heavy atoms of the structural core. The resulting structure consists of five amphipathic helices arranged in a right-handed super helix, a folding motif found in other proteins of the prolamin superfamily. As in the case of the mature protein, the recombinant precursor behaves as a plant food allergen. To trace out the origin and characteristics of its allergenic properties, rproBnIb was assayed against simulated gastric fluid and found to be very resistant to proteolysis. Also, heat treatment of the protein followed up to 85 degrees C by circular dichroism showed a very limited unfolding, which was recovered after cooling to 20 degrees C, indicating a high thermal stability. These results suggest that rproBnIb, as other 2S albumins, may be able to reach the gut immune system intact. A comparison of the putative epitopes against IgE antibodies of the three members of the prolamine family [2S albumins, nonspecific lipid transfer proteins (nsLTPs), and alpha-amylase/trypsin inhibitors] indicates that there are not common surfaces of interaction with IgE. Though the epitopes appear to be located in different regions of the proteins, they do comply with the requirements of being solvent-exposed and flexible.
- Instituto de Química Física Rocasolano, CSIC, Serrano 119, Madrid 28006, Spain.
Organizational Affiliation: