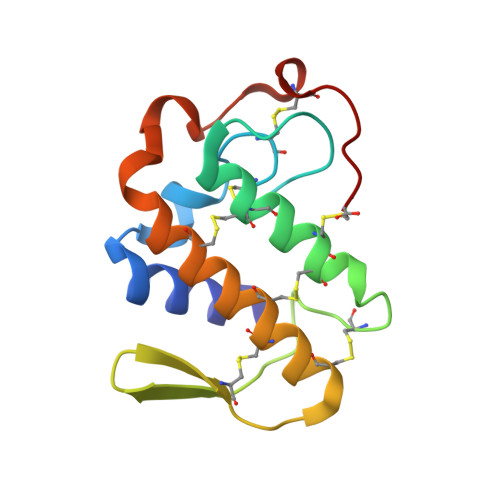
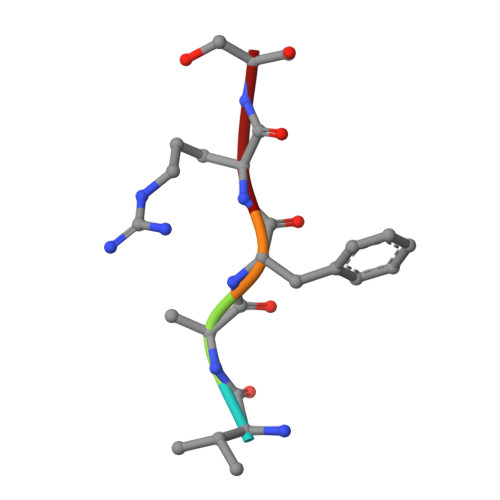
Structure-based rational drug design: Crystal structure of the complex formed between Phospholipase A2 and a pentapeptide Val-Ala-Phe-Arg-Ser
Ethayathulla, A.S., Singh, N., Sharma, S., Makker, J., Dey, S., Perbandt, M., Betzel, C., Singh, T.P.To be published.