Determination of the three-dimensional solution structure of scyllatoxin by 1H nuclear magnetic resonance.
Martins, J.C., Van de Ven, F.J., Borremans, F.A.(1995) J Mol Biology 253: 590-603
- PubMed: 7473736
- DOI: https://doi.org/10.1006/jmbi.1995.0575
- Primary Citation of Related Structures:
1SCY - PubMed Abstract:
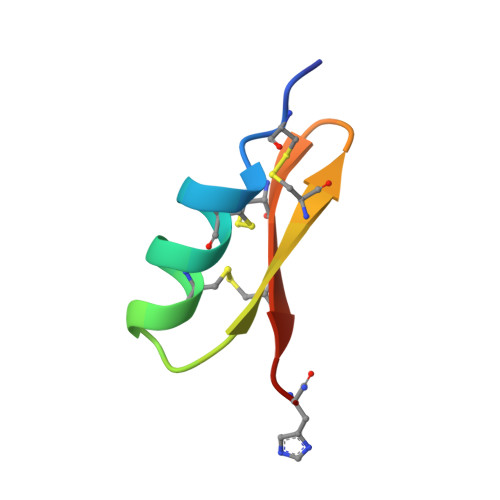
The three-dimensional solution structure of Scyllatoxin (leiurotoxin I) a venom peptide from the scorpion Leiurus quinquestriatus hebraeus was determined at 1 A resolution by homonuclear proton n.m.r. methods at 500 MHz. Data analysis and structure calculation followed conventional protocols inherent to DIANA and related programs with two exceptions. First, distance constraints were obtained from two-dimensional nuclear Overhauser spectra by a previously described partial relaxation matrix approach. Second, since the pairing pattern of the six cysteine residues was not established a priori, the unequivocal assignment of the disulfide bridges was achieved exclusively from the n.m.r. data by a statistical analysis of preliminary DIANA structures. In the final calculation we used 227 upper distance constraints, 67 torsional constraints from vicinal coupling constants and ten stereospecific assignments of beta-methylene protons. Scyllatoxin folds into a compact ellipsoidal shape. An alpha-helix (defined with 0.24 A resolution) spanning 2.5 turns from Leu5 till Ser14 is stabilized by Cys8-Cys26 and Cys12-Cys28 disulfide bridges to the carboxy-terminal strand of an anti-parallel beta-sheet. The antiparallel beta-sheet (defined at 0.66 A resolution) extends from Leu18 to Val29 with a tight turn at Gly23-Asp24 and displays a right-handed twist. Scyllatoxin competes with the toxins apamin from Apis mellifera mellifera and P05 from Androctonus mauretanicus mauretanicus for the same or similar high conductance calcium-activated potassium channels. Consideration of presently known biological activities and three-dimensional structures of these toxins suggest a different toxin-receptor interaction of scyllatoxin as compared to apamin and P05.
- Vakgebied Biomoleculaire NMR, Vakgroep Organische Chemie, Universiteit Gent, Belgium.
Organizational Affiliation: