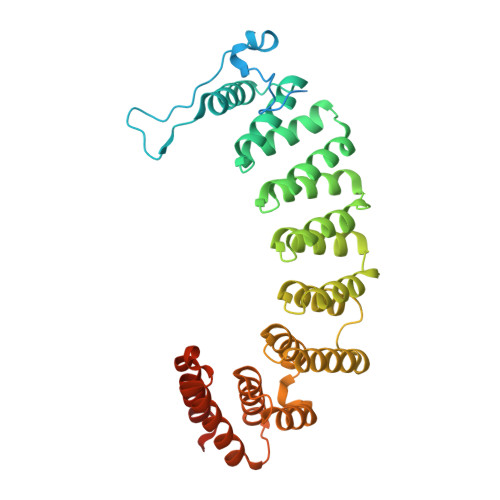
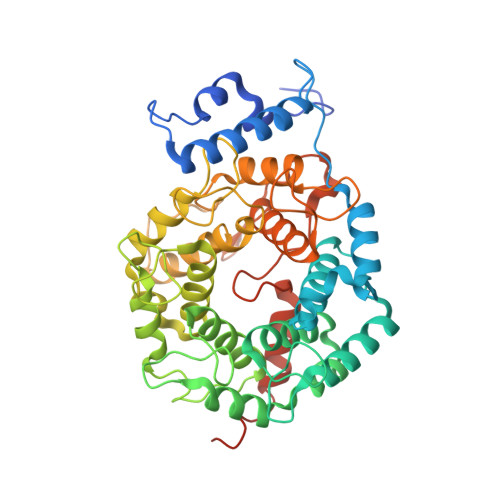
Crystal Structures of the Anticancer Clinical Candidates R115777 (Tipifarnib) and BMS-214662 Complexed with Protein Farnesyltransferase Suggest a Mechanism of FTI Selectivity.
Reid, T.S., Beese, L.S.(2004) Biochemistry 43: 6877-6884
- PubMed: 15170324
- DOI: https://doi.org/10.1021/bi049723b
- Primary Citation of Related Structures:
1SA4, 1SA5 - PubMed Abstract:
The search for new cancer therapeutics has identified protein farnesyltransferase (FTase) as a promising drug target. This enzyme attaches isoprenoid lipids to signal transduction proteins involved in growth and differentiation. The two FTase inhibitors (FTIs), R115777 (tipifarnib/Zarnestra) and BMS-214662, have undergone evaluation as cancer therapeutics in phase I and II clinical trials. R115777 has been evaluated in phase III clinical trials and shows indications for the treatment of blood and breast malignancies. Here we present crystal structures of R115777 and BMS-214662 complexed with mammalian FTase. These structures illustrate the molecular mechanism of inhibition and selectivity toward FTase over the related enzyme, protein geranylgeranyltransferase type I (GGTase-I). These results, combined with previous biochemical and structural analyses, identify features of FTase that could be exploited to modulate inhibitor potency and specificity and should aid in the continued development of FTIs as therapeutics for the treatment of cancer and parasitic infections.
- Department of Biochemistry, Duke University Medical Center, Durham, North Carolina 27710, USA.
Organizational Affiliation: