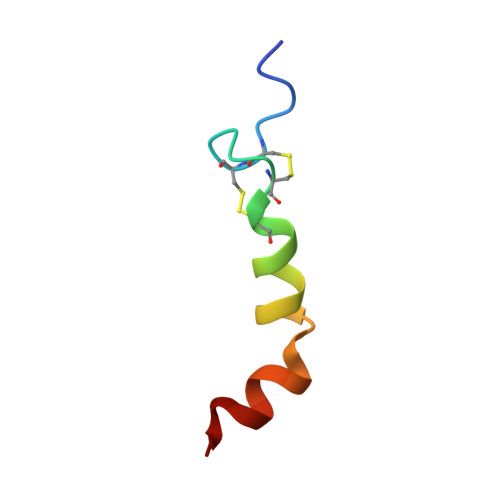
Solution structure of human orexin-A: regulator of appetite and wakefulness.
Kim, H.Y., Hong, E., Kim, J.I., Lee, W.(2004) J Biochem Mol Biol 37: 565-573
- PubMed: 15479620
- DOI: https://doi.org/10.5483/bmbrep.2004.37.5.565
- Primary Citation of Related Structures:
1R02 - PubMed Abstract:
Orexin-A and orexin-B (hypocretin-1 and hypocretin-2, respectively) are important hypothalamic neuro-peptides, which are encoded by a single mRNA transcript and stimulate food intake as well as regulate wakefulness. Here we determined the solution structure of orexin-A by NMR spectroscopy and by simulated-annealing calculation. The structural features of orexin-A involve two alpha-helices, with the hydrophobic residues disposed to on one side of helix, and hydrophilic residues to the other. A hydrophilic turn induced by two disulfide bonds provides the key difference between orexin-A and -B. With previous mutagenic studies, the derived structure of orexin-A provides us with a structure-functional view for novel drug design.
- Department of Biochemistry, College of Science, Yonsei University, Seoul 120-740, Korea.
Organizational Affiliation: